Rapid desensitization induces internalization of antigen-specific IgE on mouse mast cells
- PMID: 23810240
- PMCID: PMC3789647
- DOI: 10.1016/j.jaci.2013.05.004
Rapid desensitization induces internalization of antigen-specific IgE on mouse mast cells
Abstract
Background: Rapid desensitization transiently prevents severe allergic reactions, allowing administration of life-saving therapies in previously sensitized patients. However, the mechanisms underlying successful rapid desensitization are not fully understood.
Objectives: We sought to investigate whether the mast cell (MC) is an important target of rapid desensitization in mice sensitized to exhibit IgE-dependent passive systemic anaphylaxis in vivo and to investigate the antigen specificity and underlying mechanisms of rapid desensitization in our mouse model.
Methods: C57BL/6 mice (in vivo) or primary isolated C57BL/6 mouse peritoneal mast cells (PMCs; in vitro) were passively sensitized with antigen-specific anti-2,4-dinitrophenyl IgE, anti-ovalbumin IgE, or both. MCs were exposed over a short period of time to increasing amounts of antigen (2,4-dinitrophenyl-human serum albumin or ovalbumin) in the presence of extracellular calcium in vitro or by means of intravenous administration to sensitized mice in vivo before challenging the mice with or exposing the PMCs to optimal amounts of specific or irrelevant antigen.
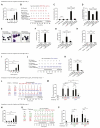
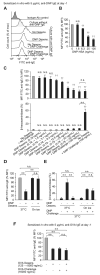
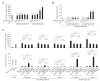
Results: Rapidly exposing mice or PMCs to progressively increasing amounts of specific antigen inhibited the development of antigen-induced hypothermia in sensitized mice in vivo and inhibited antigen-induced PMC degranulation and prostaglandin D2 synthesis in vitro. Such MC hyporesponsiveness was induced antigen-specifically and was associated with a significant reduction in antigen-specific IgE levels on MC surfaces.
Conclusions: Rapidly exposing MCs to progressively increasing amounts of antigen can both enhance the internalization of antigen-specific IgE on the MC surface and also desensitize these cells in an antigen-specific manner in vivo and in vitro.
Keywords: 2,4-Dinitrophenyl; Anaphylaxis; BMCMC; Bone marrow–derived cultured mast cell; DNP; FITC; Fluorescein isothiocyanate; HSA; Human serum albumin; IgE; MC; MCPT-1; MFI; Mast cell; Mast cell protease 1; Mean fluorescence intensity; OVA; Ovalbumin; PGD(2); PMC; PSA; Passive systemic anaphylaxis; Peritoneal mast cell; Prostaglandin D(2); STAT6; Signal transducer and activator of transcription 6; antigen; basophil; degranulation; desensitization; mast cell; rapid desensitization; receptor internalization; rush desensitization.
Copyright © 2013 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures




References
-
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. - PubMed
-
- Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402:B24–30. - PubMed
-
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–26. - PubMed
-
- Ring J, Grosber M, Mohrenschlager M, Brockow K. Anaphylaxis: acute treatment and management. Chem Immunol Allergy. 2010;95:201–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

