SHORT syndrome with partial lipodystrophy due to impaired phosphatidylinositol 3 kinase signaling
- PMID: 23810379
- PMCID: PMC3710758
- DOI: 10.1016/j.ajhg.2013.05.023
SHORT syndrome with partial lipodystrophy due to impaired phosphatidylinositol 3 kinase signaling
Abstract
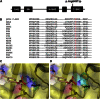
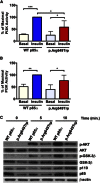
The phosphatidylinositol 3 kinase (PI3K) pathway regulates fundamental cellular processes such as metabolism, proliferation, and survival. A central component in this pathway is the p85α regulatory subunit, encoded by PIK3R1. Using whole-exome sequencing, we identified a heterozygous PIK3R1 mutation (c.1945C>T [p.Arg649Trp]) in two unrelated families affected by partial lipodystrophy, low body mass index, short stature, progeroid face, and Rieger anomaly (SHORT syndrome). This mutation led to impaired interaction between p85α and IRS-1 and reduced AKT-mediated insulin signaling in fibroblasts from affected subjects and in reconstituted Pik3r1-knockout preadipocytes. Normal PI3K activity is critical for adipose differentiation and insulin signaling; the mutated PIK3R1 therefore provides a unique link among lipodystrophy, growth, and insulin signaling.
Copyright © 2013 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Aubin D., Gagnon A., Sorisky A. Phosphoinositide 3-kinase is required for human adipocyte differentiation in culture. Int J Obes (Lond) 2005;29:1006–1009. - PubMed
-
- Engelman J.A., Luo J., Cantley L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. - PubMed
-
- Ueki K., Fruman D.A., Yballe C.M., Fasshauer M., Klein J., Asano T., Cantley L.C., Kahn C.R. Positive and negative roles of p85 alpha and p85 beta regulatory subunits of phosphoinositide 3-kinase in insulin signaling. J. Biol. Chem. 2003;278:48453–48466. - PubMed
-
- Taniguchi C.M., Emanuelli B., Kahn C.R. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006;7:85–96. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

