Differential innervation of direct- and indirect-pathway striatal projection neurons
- PMID: 23810541
- PMCID: PMC3729794
- DOI: 10.1016/j.neuron.2013.05.014
Differential innervation of direct- and indirect-pathway striatal projection neurons
Abstract
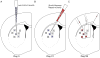
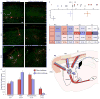
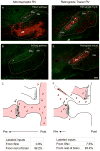
The striatum integrates information from multiple brain regions to shape motor learning. The two major projection cell types in striatum target different downstream basal ganglia targets and have opposing effects on motivated behavior, yet differential innervation of these neuronal subtypes is not well understood. To examine whether input specificity provides a substrate for information segregation in these circuits, we used a monosynaptic rabies virus system to generate brain-wide maps of neurons that form synapses with direct- or indirect-pathway striatal projection neurons. We discovered that sensory cortical and limbic structures preferentially innervated the direct pathway, whereas motor cortex preferentially targeted the indirect pathway. Thalamostriatal input, dopaminergic input, as well as input from specific cortical layers, was similar onto both pathways. We also confirm synaptic innervation of striatal projection neurons by the raphe and pedunculopontine nuclei. Together, these findings provide a framework for guiding future studies of basal ganglia circuit function.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Arbuthnott GW, Wickens J. Space, time and dopamine. Trends Neurosci. 2007;30:62–69. - PubMed
-
- Ballion B, Mallet N, Bezard E, Lanciego JL, Gonon F. Intratelencephalic corticostriatal neurons equally excite striatonigral and striatopallidal neurons and their discharge activity is selectively reduced in experimental parkinsonism. Eur J Neurosci. 2008;27:2313–2321. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

