Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen
- PMID: 23810578
- PMCID: PMC3743101
- DOI: 10.1016/j.jaut.2013.06.003
Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen
Abstract
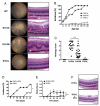
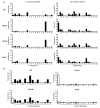
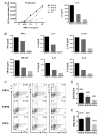
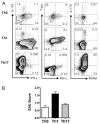
Despite presence of circulating retina-specific T cells in healthy individuals, ocular immune privilege usually averts development of autoimmune uveitis. To study the breakdown of immune privilege and development of disease, we generated transgenic (Tg) mice that express a T cell receptor (TCR) specific for interphotoreceptor retinoid-binding protein (IRBP), which serves as an autoimmune target in uveitis induced by immunization. Three lines of TCR Tg mice, with different levels of expression of the transgenic R161 TCR and different proportions of IRBP-specific CD4⁺ T cells in their peripheral repertoire, were successfully established. Importantly, two of the lines rapidly developed spontaneous uveitis, reaching 100% incidence by 2 and 3 months of age, respectively, whereas the third appeared "poised" and only developed appreciable disease upon immune perturbation. Susceptibility roughly paralleled expression of the R161 TCR. In all three lines, peripheral CD4⁺ T cells displayed a naïve phenotype, but proliferated in vitro in response to IRBP and elicited uveitis upon adoptive transfer. In contrast, CD4⁺ T cells infiltrating uveitic eyes mostly showed an effector/memory phenotype, and included Th1, Th17 as well as T regulatory cells that appeared to have been peripherally converted from conventional CD4⁺ T cells rather than thymically derived. Thus, R161 mice provide a new and valuable model of spontaneous autoimmune disease that circumvents the limitations of active immunization and adjuvants, and allows to study basic mechanisms involved in maintenance and breakdown of immune homeostasis affecting immunologically privileged sites such as the eye.
Keywords: AIRE; Ag; Autoimmune uveitis; CFA; EAU; HEL; IRBP; Immune privilege; PMA; RAG; SP; Spontaneous disease; T cell receptor; TCR; TCR transgenic mouse; WT; antigen; autoimmune regulator; complete Freund's adjuvant; experimental autoimmune uveitis; hen egg lysozyme; interphotoreceptor retinoid binding protein; phorbol myristate acetate; recombination activating gene; single positive; wild type.
Published by Elsevier Ltd.
Figures



References
-
- Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74:179–85. - PubMed
-
- Caspi RR. Ocular autoimmunity: the price of privilege? Immunol Rev. 2006;213:23–35. - PubMed
-
- Nussenblatt RB, Whitcup SM. Uveitis: fundamentals and clinical practice. 3rd ed. Elsevier; Philadelphia, PA: 2004. Mosby.
-
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. - PubMed
-
- Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218:223–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

