Long-term effects of cocaine experience on neuroplasticity in the nucleus accumbens core of addiction-prone rats
- PMID: 23811073
- PMCID: PMC3859827
- DOI: 10.1016/j.neuroscience.2013.06.042
Long-term effects of cocaine experience on neuroplasticity in the nucleus accumbens core of addiction-prone rats
Abstract
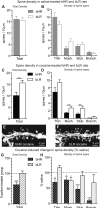
Repeated exposure to drugs of abuse is associated with structural plasticity in brain reward pathways. Rats selectively bred for locomotor response to novelty differ on a number of neurobehavioral dimensions relevant to addiction. This unique genetic animal model was used here to examine both pre-existing differences and long-term consequences of repeated cocaine treatment on structural plasticity. Selectively bred high-responder (bHR) and low-responder (bLR) rats received repeated saline or cocaine injections for 9 consecutive days. Escalating doses of cocaine (7.5, 15 and 30 mg/kg) were administered on the first (day 1) and last (day 9) days of treatment and a single injection of the intermediate dose (15 mg/kg) was given on days 2-8. Motor activity in response to escalating doses of cocaine was compared on the first and last days of treatment to assess the acute and sensitized response to the drug. Following prolonged cocaine abstinence (28 days), spine density was examined on terminal dendrites of medium spiny neurons in the nucleus accumbens core. Relative to bLRs, bHRs exhibited increased psychomotor activation in response to both the acute and repeated effects of cocaine. There were no differences in spine density between bHR and bLR rats under basal conditions or following repeated saline treatment. However, spine density differed markedly between these two lines following prolonged cocaine abstinence. All spine types were decreased in cocaine-treated bHRs, while only mushroom spines were decreased in bLRs that received cocaine. Changes in spine density occurred specifically near the branch point of terminal dendrites. These findings indicate that structural plasticity associated with prolonged cocaine abstinence varies markedly in two selected strains of rats that vary on numerous traits relevant to addiction. Thus, genetic factors that contribute to individual variation in the behavioral response to cocaine also influence cocaine-induced structural plasticity.
Keywords: addiction; cocaine; dendrites; prolonged abstinence; psychomotor sensitization; spine density.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures


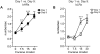

References
-
- Ahmed SH, Stinus L, Le Moal M, Cador M. Controlling interindividual differences in the unconditioned response to amphetamine in the study of environment-dependent sensitization. Behav Pharmacol. 1993;4:355–365. - PubMed
-
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

