Nicotinic acetylcholine receptors containing the α6 subunit contribute to ethanol activation of ventral tegmental area dopaminergic neurons
- PMID: 23811312
- PMCID: PMC3842157
- DOI: 10.1016/j.bcp.2013.06.015
Nicotinic acetylcholine receptors containing the α6 subunit contribute to ethanol activation of ventral tegmental area dopaminergic neurons
Abstract
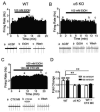
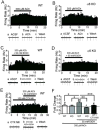
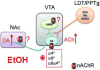
Nicotine and alcohol are often co-abused suggesting a common mechanism of action may underlie their reinforcing properties. Both drugs acutely increase activity of ventral tegmental area (VTA) dopaminergic (DAergic) neurons, a phenomenon associated with reward behavior. Recent evidence indicates that nicotinic acetylcholine receptors (nAChRs), ligand-gated cation channels activated by ACh and nicotine, may contribute to ethanol-mediated activation of VTA DAergic neurons although the nAChR subtype(s) involved has not been fully elucidated. Here we show that expression and activation of nAChRs containing the α6 subunit contribute to ethanol-induced activation of VTA DAergic neurons. In wild-type (WT) mouse midbrain sections that contain the VTA, ethanol (50 or 100 mM) significantly increased firing frequency of DAergic neurons. In contrast, ethanol did not significantly increase activity of VTA DAergic neurons in mice that do not express CHRNA6, the gene encoding the α6 nAChR subunit (α6 knock-out (KO) mice). Ethanol-induced activity in WT slices was also reduced by pre-application of the α6 subtype-selective nAChR antagonist, α-conotoxin MII[E11A]. When co-applied, ethanol potentiated the response to ACh in WT DAergic neurons; whereas co-application of ACh and ethanol failed to significantly increase activity of DAergic neurons in α6 KO slices. Finally, pre-application of α-conotoxin MII[E11A] in WT slices reduced ethanol potentiation of ACh responses. Together our data indicate that α6-subunit containing nAChRs may contribute to ethanol activation of VTA DAergic neurons. These receptors are predominantly expressed in DAergic neurons and known to be critical for nicotine reinforcement, providing a potential common therapeutic molecular target to reduce nicotine and alcohol co-abuse.
Keywords: Acetylcholine; Alcoholism; Dopamine; Nicotinic receptor; Ventral tegmental area.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest: None
Figures

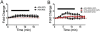
References
-
- Batel P, Pessione F, Maitre C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977–80. - PubMed
-
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

