Theoretical principles underlying optical stimulation of myelinated axons expressing channelrhodopsin-2
- PMID: 23811392
- PMCID: PMC4116477
- DOI: 10.1016/j.neuroscience.2013.06.031
Theoretical principles underlying optical stimulation of myelinated axons expressing channelrhodopsin-2
Abstract
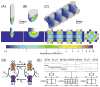
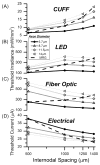
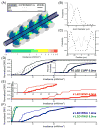
Numerous clinical conditions can be treated by neuromodulation of the peripheral nervous system (PNS). Typical electrical PNS therapies activate large diameter axons at lower electrical stimulus thresholds than small diameter axons. However, recent animal experiments with peripheral optogenetic neural stimulation (PONS) of myelinated axons expressing channelrhodopsin-2 (ChR2) have shown that this technique activates small diameter axons at lower irradiances than large diameter axons. We hypothesized that the small-to-large diameter recruitment order primarily arises from the internodal spacing relationship of myelinated axons. Small diameter axons have shorter distances between their nodes of Ranvier, which increases the number of nodes of Ranvier directly illuminated relative to larger diameter axons. We constructed "light-axon" PONS models that included multi-compartment, double cable, myelinated axon models embedded with ChR2 membrane dynamics, coupled with a model of blue light dynamics in the tissue medium from a range of different light sources. The light-axon models enabled direct calculation of threshold irradiance for different diameter axons. Our simulations demonstrate that illumination of multiple nodal sections reduces the threshold irradiance and enhances the small-to-large diameter recruitment order. In addition to addressing biophysical questions, our light-axon model system could also be useful in guiding the engineering design of optical stimulation technology that could maximize the efficiency and selectivity of PONS.
Keywords: ChR2; action potential; node of Ranvier; optogenetic.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests related to this work.
Figures




References
-
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–S156. - PubMed
-
- Grossman N, Nikolic K, Toumazou C, Degenaar P. Modeling study of the light stimulation of a neuron cell with channelrhodopsin-2 mutants. IEEE Trans Biomed Eng. 2011;58:1742–1751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

