Persistence of antibodies in adolescents 18-24 months after immunization with one, two, or three doses of 4CMenB meningococcal serogroup B vaccine
- PMID: 23811804
- PMCID: PMC3981837
- DOI: 10.4161/hv.25505
Persistence of antibodies in adolescents 18-24 months after immunization with one, two, or three doses of 4CMenB meningococcal serogroup B vaccine
Abstract
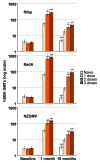
We previously demonstrated the immunogenicity and tolerability of the serogroup B meningococcal vaccine, 4CMenB (Bexsero), in 11-17 y-olds randomized to receive 1, 2, or 3 doses at 1, 2, or 6 mo intervals. Participants in this extension study provided an additional blood sample 18-24 mo after last vaccine dose, to assess persistence of serum bactericidal activity with human complement (hSBA), and to compare with age-matched 4CMenB-naïve controls. In the original study, one month after one 4CMenB dose, 93% of subjects had seroprotective hSBA titers (≥4) against indicator serogroup B strains for individual vaccine antigens (fHbp, NadA and NZOMV), increasing to ~100% after two or three doses. After 18-24 mo, 62-73% of subjects given one dose had titers ≥4 against the three antigens, significantly lower rates than after two (77-94%) or three (86-97%) doses. Only proportions with titers ≥ 4 against NZOMV were significantly different between the two (77%) and three (90%, p < 0.0001) dose groups. These results confirm that two doses of 4CMenB, administered 1 to 6 mo apart, provide good levels of bactericidal activity against serogroup B meningococci, which were sustained at least 18-24 mo in over 64% of adolescents for all three tested vaccine-related antigens.
Keywords: adolescents; antibodies; meningococcal; persistence; serogroup B; vaccine.
Figures
References
-
- Jennings HJ, Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981;127:1011–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
