Identification of PTEN at the ER and MAMs and its regulation of Ca(2+) signaling and apoptosis in a protein phosphatase-dependent manner
- PMID: 23811847
- PMCID: PMC3824603
- DOI: 10.1038/cdd.2013.77
Identification of PTEN at the ER and MAMs and its regulation of Ca(2+) signaling and apoptosis in a protein phosphatase-dependent manner
Abstract
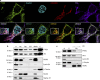
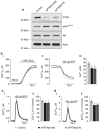
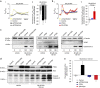
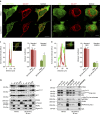
The tumor suppressor activity of PTEN (phosphatase and tensin homolog deleted on chromosome 10) is thought to be largely attributable to its lipid phosphatase activity. PTEN dephosphorylates the lipid second messenger phosphatidylinositol 3,4,5-trisphosphate to directly antagonize the phosphoinositide 3-kinase-Akt pathway and prevent the activating phosphorylation of Akt. PTEN has also other proposed mechanisms of action, including a poorly characterized protein phosphatase activity, protein-protein interactions, as well as emerging functions in different compartment of the cells such as nucleus and mitochondria. We show here that a fraction of PTEN protein localizes to the endoplasmic reticulum (ER) and mitochondria-associated membranes (MAMs), signaling domains involved in calcium ((2+)) transfer from the ER to mitochondria and apoptosis induction. We demonstrate that PTEN silencing impairs ER Ca(2+) release, lowers cytosolic and mitochondrial Ca(2+) transients and decreases cellular sensitivity to Ca(2+)-mediated apoptotic stimulation. Specific targeting of PTEN to the ER is sufficient to enhance ER-to-mitochondria Ca(2+) transfer and sensitivity to apoptosis. PTEN localization at the ER is further increased during Ca(2+)-dependent apoptosis induction. Importantly, PTEN interacts with the inositol 1,4,5-trisphosphate receptors (IP3Rs) and this correlates with the reduction in their phosphorylation and increased Ca(2+) release. We propose that ER-localized PTEN regulates Ca(2+) release from the ER in a protein phosphatase-dependent manner that counteracts Akt-mediated reduction in Ca(2+) release via IP3Rs. These findings provide new insights into the mechanisms and the extent of PTEN tumor-suppressive functions, highlighting new potential strategies for therapeutic intervention.
Figures

References
-
- Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. - PubMed
-
- Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–198. - PubMed
-
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. - PubMed
-
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

