Agonists of MAS oncogene and angiotensin II type 2 receptors attenuate cardiopulmonary disease in rats with neonatal hyperoxia-induced lung injury
- PMID: 23812633
- PMCID: PMC3763032
- DOI: 10.1152/ajplung.00360.2012
Agonists of MAS oncogene and angiotensin II type 2 receptors attenuate cardiopulmonary disease in rats with neonatal hyperoxia-induced lung injury
Abstract
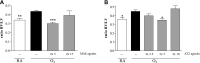
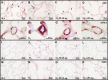
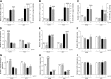
Stimulation of MAS oncogene receptor (MAS) or angiotensin (Ang) receptor type 2 (AT2) may be novel therapeutic options for neonatal chronic lung disease (CLD) by counterbalancing the adverse effects of the potent vasoconstrictor angiotensin II, consisting of arterial hypertension (PAH)-induced right ventricular hypertrophy (RVH) and pulmonary inflammation. We determined the cardiopulmonary effects in neonatal rats with CLD of daily treatment during continuous exposure to 100% oxygen for 10 days with specific ligands for MAS [cyclic Ang-(1-7); 10-50 μg·kg(-1)·day(-1)] and AT2 [dKcAng-(1-7); 5-20 μg·kg(-1)·day(-1)]. Parameters investigated included lung and heart histopathology, fibrin deposition, vascular leakage, and differential mRNA expression in the lungs of key genes involved in the renin-angiotensin system, inflammation, coagulation, and alveolar development. We investigated the role of nitric oxide synthase inhibition with N(ω)-nitro-l-arginine methyl ester (25 mg·kg(-1)·day(-1)) during AT2 agonist treatment. Prophylactic treatment with agonists for MAS or AT2 for 10 days diminished cardiopulmonary injury by reducing alveolar septum thickness and medial wall thickness of small arterioles and preventing RVH. Both agonists attenuated the pulmonary influx of inflammatory cells, including macrophages (via AT2) and neutrophils (via MAS) but did not reduce alveolar enlargement and vascular alveolar leakage. The AT2 agonist attenuated hyperoxia-induced fibrin deposition. In conclusion, stimulation of MAS or AT2 attenuates cardiopulmonary injury by reducing pulmonary inflammation and preventing PAH-induced RVH but does not affect alveolar and vascular development in neonatal rats with experimental CLD. The beneficial effects of AT2 activation on experimental CLD were mediated via a NOS-independent mechanism.
Keywords: angiotensin-(1-7); bronchopulmonary dysplasia; lung inflammation; pulmonary hypertension; right ventricular hypertrophy.
Figures





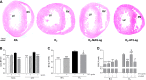
References
-
- Abman SH. Recent advances in the pathogenesis and treatment of persistent pulmonary hypertension of the newborn. Neonatology 91: 283–290, 2007 - PubMed
-
- Abman SH. Role of endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Annu Rev Med 60: 13–23, 2009 - PubMed
-
- Adachi Y, Saito Y, Kishimoto I, Harada M, Kuwahara K, Takahashi N, Kawakami R, Nakanishi M, Nakagawa Y, Tanimoto K, Saitoh Y, Yasuno S, Usami S, Iwai M, Horiuchi M, Nakao K. Angiotensin II type 2 receptor deficiency exacerbates heart failure and reduces survival after acute myocardial infarction in mice. Circulation 107: 2406–2408, 2003 - PubMed
-
- Alenina N, Xu P, Rentzsh B, Patkin EL, Bader M. Genetically altered animal models for MAS and angiotensin-(1-7). Exp Physiol 93: 528–537, 2008 - PubMed
-
- Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 357: 1946–1955, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

