Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome
- PMID: 23813218
- PMCID: PMC3785276
- DOI: 10.1681/ASN.2012121200
Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome
Abstract
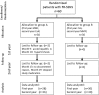
The severe side effects of long-term corticosteroid or cyclosporin A (CsA) therapy complicate the treatment of children with frequently relapsing steroid-sensitive nephrotic syndrome (FR-SSNS). We conducted a randomized, multicenter, open-label, crossover study comparing the efficacy and safety of a 1-year treatment with mycophenolate mofetil (MMF; target plasma mycophenolic acid trough level of 1.5-2.5 µg/ml) or CsA (target trough level of 80-100 ng/ml) in 60 pediatric patients with FR-SSNS. We assessed the frequency of relapse as the primary endpoint and evaluated pharmacokinetic profiles (area under the curve [AUC]) after 3 and 6 months of treatment. More relapses per patient per year occurred with MMF than with CsA during the first year (P=0.03), but not during the second year (P=0.14). No relapses occurred in 85% of patients during CsA therapy and in 64% of patients during MMF therapy (P=0.06). However, the time without relapse was significantly longer with CsA than with MMF during the first year (P<0.05), but not during the second year (P=0.36). In post hoc analysis, patients with low mycophenolic acid exposure (AUC <50 µg⋅h/ml) experienced 1.4 relapses per year compared with 0.27 relapses per year in those with high exposure (AUC>50 µg⋅h/ml; P<0.05). There were no significant differences between groups with respect to BP, growth, lipid levels, or adverse events. However, cystatin clearance, estimated GFR, and hemoglobin levels increased significantly with MMF compared with CsA. These results indicate that MMF is inferior to CsA in preventing relapses in pediatric patients with FR-SSNS, but may be a less nephrotoxic treatment option.
Figures


References
-
- International Study of Kidney Disease in Children : The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981 - PubMed
-
- Hodson EM, Willis NS, Craig JC: Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev (4): CD001533, 2007 - PubMed
-
- Tarshish P, Tobin JN, Bernstein J, Edelmann CM, Jr: Prognostic significance of the early course of minimal change nephrotic syndrome: Report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8: 769–776, 1997 - PubMed
-
- Hodson EM, Willis NS, Craig JC: Non-corticosteroid treatment for nephrotic syndrome in children. Cochrane Database Syst Rev (1): CD002290, 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

