A novel role of nucleostemin in maintaining the genome integrity of dividing hepatocytes during mouse liver development and regeneration
- PMID: 23813570
- PMCID: PMC3844114
- DOI: 10.1002/hep.26600
A novel role of nucleostemin in maintaining the genome integrity of dividing hepatocytes during mouse liver development and regeneration
Abstract
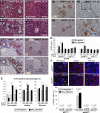
During liver development and regeneration, hepatocytes undergo rapid cell division and face an increased risk of DNA damage associated with active DNA replication. The mechanism that protects proliferating hepatocytes from replication-induced DNA damage remains unclear. Nucleostemin (NS) is known to be up-regulated during liver regeneration, and loss of NS is associated with increased DNA damage in cancer cells. To determine whether NS is involved in protecting the genome integrity of proliferating hepatocytes, we created an albumin promoter-driven NS conditional-null (albNS(cko) ) mouse model. Livers of albNS(cko) mice begin to show loss of NS in developing hepatocytes from the first postnatal week and increased DNA damage and hepatocellular injury at 1-2 weeks of age. At 3-4 weeks, albNS(cko) livers develop bile duct hyperplasia and show increased apoptotic cells, necrosis, regenerative nodules, and evidence suggestive of hepatic stem/progenitor cell activation. CCl4 treatment enhances degeneration and DNA damage in NS-deleted hepatocytes and increases biliary hyperplasia and A6(+) cells in albNS(cko) livers. After 70% partial hepatectomy, albNS(cko) livers show increased DNA damage in parallel with a blunted and prolonged regenerative response. The DNA damage in NS-depleted hepatocytes is explained by the impaired recruitment of a core DNA repair enzyme, RAD51, to replication-induced DNA damage foci.
Conclusion: This work reveals a novel genome-protective role of NS in developing and regenerating hepatocytes.
Copyright © 2013 by the American Association for the Study of Liver Diseases.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
