Vascular risk factors: a ticking time bomb to Alzheimer's disease
- PMID: 23813612
- PMCID: PMC10852736
- DOI: 10.1177/1533317513494457
Vascular risk factors: a ticking time bomb to Alzheimer's disease
Abstract
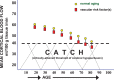
Evidence is growing that vascular risk factors (VRFs) for Alzheimer's disease (AD) affect cerebral hemodynamics to launch a cascade of cellular and molecular changes that initiate cognitive deficits and eventual progression of AD. Neuroimaging studies have reported VRFs for AD to be accurate predictors of cognitive decline and dementia. In regions that participate in higher cognitive function, middle temporal, posterior cingulate, inferior parietal and precuneus regions, and neuroimaging studies indicate an association involving VRFs, cerebral hypoperfusion, and cognitive decline in elderly individuals who develop AD. The VRF can be present in cognitively intact individuals for decades before mild cognitive deficits or neuropathological signs are manifested. In that sense, they may be "ticking time bombs" before cognitive function is demolished. Preventive intervention of modifiable VRF may delay or block progression of AD. Intervention could target cerebral blood flow (CBF), since most VRFs act to lower CBF in aging individuals by promoting cerebrovascular dysfunction.
Keywords: Alzheimer’s; aging; cerebral blood flow; hypoperfusion; neuroimaging; vascular risk factors.
Conflict of interest statement
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Buxton R. Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques. Cambridge, England: Cambridge University Press; 2002.
-
- Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 2009;113(pt 1):27–47. - PubMed
-
- Pantano P, Baron JC, Lebrun-Grandie P, Duquesnoy N, Bousser MG, Comar D. Regional cerebral blood flow and oxygen consumption in human aging. Stroke. 1994;15(4):635–641. - PubMed
-
- Gur RC, Gur RE, Obrist WD, Skolnick BE, Reivich M. Age and regional cerebral blood flow at rest and during cognitive activity. Arch Gen Psychiatry. 1987;44(7):617–621. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

