Effect of a nurse-coordinated prevention programme on cardiovascular risk after an acute coronary syndrome: main results of the RESPONSE randomised trial
- PMID: 23813851
- PMCID: PMC3786610
- DOI: 10.1136/heartjnl-2013-303989
Effect of a nurse-coordinated prevention programme on cardiovascular risk after an acute coronary syndrome: main results of the RESPONSE randomised trial
Abstract
Objective: To quantify the impact of a practical, hospital-based nurse-coordinated prevention programme on cardiovascular risk, integrated into the routine clinical care of patients discharged after an acute coronary syndrome, as compared with usual care only.
Design: RESPONSE (Randomised Evaluation of Secondary Prevention by Outpatient Nurse SpEcialists) was a randomised clinical trial.
Setting: Multicentre trial in secondary and tertiary healthcare settings.
Participants: 754 patients admitted for acute coronary syndrome.
Intervention: A nurse-coordinated prevention programme, consisting of four outpatient nurse clinic visits, focusing on healthy lifestyles, biometric risk factors and medication adherence, in addition to usual care.
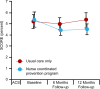
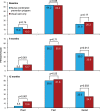
Main outcome measures: The main outcome was 10-year cardiovascular mortality risk as estimated by Systematic Coronary Risk Evaluation at 12 months follow-up. Secondary outcomes included Framingham Coronary Risk Score at 12 months, in addition to changes in individual risk factors. Risk factor control was classified as 'poor' if 0 to 3 factors were on target, 'fair' if 4 to 6 factors were on target, and 'good' if 7 to 9 were on target.
Results: The mean Systematic Coronary Risk Evaluation at 12 months was 4.4 per cent (SD 4.5) in the intervention group and 5.4 per cent (SD 6.2) in the control group (p=0.021), representing a 17.4% relative risk reduction. At 12 months, risk factor control classified as 'good' was achieved in 35% of patients in the intervention group compared with 25% in the control group (p=0.003). Attendance to the nurse-coordinated prevention programme was 92%. In the intervention group, 86 rehospitalisations were observed against 132 in the control group (relative risk reduction 34.8%, p=0.023).
Conclusions: The nurse-coordinated hospital-based prevention programme in addition to usual care is a practical, yet effective method for reduction of cardiovascular risk in patients with coronary disease. Our data suggest that the counselling component of the programme may lead to a reduction in hospital readmissions.
Trial registration trialregisternl identifier: TC1290.
Keywords: Coronary Artery Disease.
Figures

References
-
- Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006;113:2363–72 - PubMed
-
- De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: third joint task force of European and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of eight societies and by invited experts). Eur J Cardiovasc Prev Rehabil 2003;10:S1–S10 - PubMed
-
- Kotseva K, Wood D, De Backer G, et al. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil 2009;16:121–37 - PubMed
-
- Wood DA, Kotseva K, Connolly S, et al. Nurse-coordinated multidisciplinary, family-based cardiovascular disease prevention programme (EUROACTION) for patients with coronary heart disease and asymptomatic individuals at high risk of cardiovascular disease: a paired, cluster-randomised controlled trial. Lancet 2008;371:1999–2012 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
