ZO-1 recruitment to α-catenin--a novel mechanism for coupling the assembly of tight junctions to adherens junctions
- PMID: 23813953
- PMCID: PMC3757330
- DOI: 10.1242/jcs.126565
ZO-1 recruitment to α-catenin--a novel mechanism for coupling the assembly of tight junctions to adherens junctions
Abstract
The formation of a barrier between epithelial cells is a fundamental determinant of cellular homeostasis, protecting underlying cells against pathogens, dehydration and damage. Assembly of the tight junction barrier is dependent upon neighboring epithelial cells binding to one another and forming adherens junctions, but the mechanism for how these processes are linked is poorly understood. Using a knockdown and substitution system, we studied whether ZO-1 binding to α-catenin is required for coupling tight junction assembly to the formation of adherens junctions. We found that preventing ZO-1 binding to α-catenin did not appear to affect adherens junctions. Rather the assembly and maintenance of the epithelial barrier were disrupted. This disruption was accompanied by alterations in the mobility of ZO-1 and the organization of the actin cytoskeleton. Thus, our study identifies α-catenin binding to ZO-1 as a new mechanism for coupling the assembly of the epithelial barrier to cell-to-cell adhesion.
Keywords: Adherens junction; Tight junction; ZO-1; α-Catenin.
Figures
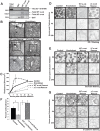
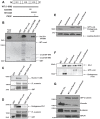


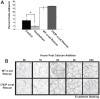
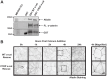
References
-
- Aberle H., Butz S., Stappert J., Weissig H., Kemler R., Hoschuetzky H. (1994). Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 107, 3655–3663 - PubMed
-
- Ando-Akatsuka Y., Yonemura S., Itoh M., Furuse M., Tsukita S. (1999). Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J. Cell. Physiol. 179, 115–125 10.1002/(SICI)1097-4652(199905)179:2<115::AID-JCP1>3.0.CO;2-T - DOI - PubMed
-
- Asakura T., Nakanishi H., Sakisaka T., Takahashi K., Mandai K., Nishimura M., Sasaki T., Takai Y. (1999). Similar and differential behaviour between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells 4, 573–581 10.1046/j.1365-2443.1999.00283.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

