Integrated phosphoproteomic and metabolomic profiling reveals NPM-ALK-mediated phosphorylation of PKM2 and metabolic reprogramming in anaplastic large cell lymphoma
- PMID: 23814019
- PMCID: PMC3739039
- DOI: 10.1182/blood-2013-01-482026
Integrated phosphoproteomic and metabolomic profiling reveals NPM-ALK-mediated phosphorylation of PKM2 and metabolic reprogramming in anaplastic large cell lymphoma
Abstract
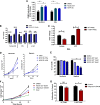
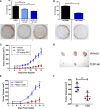
The mechanisms underlying the pathogenesis of the constitutively active tyrosine kinase nucleophosmin-anaplastic lymphoma kinase (NPM-ALK) expressing anaplastic large cell lymphoma are not completely understood. Here we show using an integrated phosphoproteomic and metabolomic strategy that NPM-ALK induces a metabolic shift toward aerobic glycolysis, increased lactate production, and biomass production. The metabolic shift is mediated through the anaplastic lymphoma kinase (ALK) phosphorylation of the tumor-specific isoform of pyruvate kinase (PKM2) at Y105, resulting in decreased enzymatic activity. Small molecule activation of PKM2 or expression of Y105F PKM2 mutant leads to reversal of the metabolic switch with increased oxidative phosphorylation and reduced lactate production coincident with increased cell death, decreased colony formation, and reduced tumor growth in an in vivo xenograft model. This study provides comprehensive profiling of the phosphoproteomic and metabolomic consequences of NPM-ALK expression and reveals a novel role of ALK in the regulation of multiple components of cellular metabolism. Our studies show that PKM2 is a novel substrate of ALK and plays a critical role in mediating the metabolic shift toward biomass production and tumorigenesis.
Figures
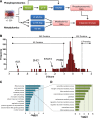

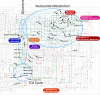
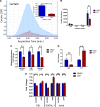
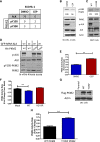
References
-
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. - PubMed
-
- Janoueix-Lerosey I, Lequin D, Brugières L, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455(7215):967–970. - PubMed
-
- Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59(12):2776–2780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

