Altered 2-thiouridylation impairs mitochondrial translation in reversible infantile respiratory chain deficiency
- PMID: 23814040
- PMCID: PMC3889809
- DOI: 10.1093/hmg/ddt309
Altered 2-thiouridylation impairs mitochondrial translation in reversible infantile respiratory chain deficiency
Abstract
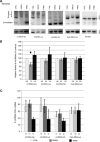
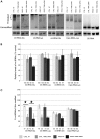
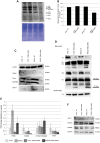
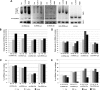
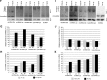
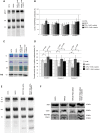
Childhood-onset mitochondrial encephalomyopathies are severe, relentlessly progressive conditions. However, reversible infantile respiratory chain deficiency (RIRCD), due to a homoplasmic mt-tRNA(Glu) mutation, and reversible infantile hepatopathy, due to tRNA 5-methylaminomethyl-2-thiouridylate methyltransferase (TRMU) deficiency, stand out by showing spontaneous recovery, and provide the key to treatments of potential broader relevance. Modification of mt-tRNA(Glu) is a possible functional link between these two conditions, since TRMU is responsible for 2-thiouridylation of mt-tRNA(Glu), mt-tRNA(Lys) and mt-tRNA(Gln). Here we show that down-regulation of TRMU in RIRCD impairs 2-thiouridylation and exacerbates the effect of the mt-tRNA(Glu) mutation by triggering a mitochondrial translation defect in vitro. Skeletal muscle of RIRCD patients in the symptomatic phase showed significantly reduced 2-thiouridylation. Supplementation with l-cysteine, which is required for optimal TRMU function, rescued respiratory chain enzyme activities in human cell lines of patients with RIRCD as well as deficient TRMU. Our results show that l-cysteine supplementation is a potential treatment for RIRCD and for TRMU deficiency, and is likely to have broader application for the growing group of intra-mitochondrial translation disorders.
Figures


Comment in
-
Neurodegenerative disease: defective mitochondrial dynamics in the hot seat-a therapeutic target common to many neurological disorders?Nat Rev Neurol. 2013 Aug;9(8):417. doi: 10.1038/nrneurol.2013.138. Epub 2013 Jul 23. Nat Rev Neurol. 2013. PMID: 23877644 No abstract available.
References
-
- Tuppen H.A., Blakely E.L., Turnbull D.M., Taylor R.W. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta. 2010;1797:113–128. - PubMed
-
- Ylikallio E., Suomalainen A. Mechanisms of mitochondrial diseases. Ann. Med. 2012;44:41–59. - PubMed
-
- Mimaki M., Hatakeyama H., Komaki H., Yokoyama M., Arai H., Kirino Y., Suzuki T., Nishino I., Nonaka I., Goto Y. Reversible infantile respiratory chain deficiency: a clinical and molecular study. Ann. Neurol. 2010;68:845–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

