Health-related quality of life with adjuvant docetaxel- and trastuzumab-based regimens in patients with node-positive and high-risk node-negative, HER2-positive early breast cancer: results from the BCIRG 006 Study
- PMID: 23814044
- PMCID: PMC3720634
- DOI: 10.1634/theoncologist.2013-0091
Health-related quality of life with adjuvant docetaxel- and trastuzumab-based regimens in patients with node-positive and high-risk node-negative, HER2-positive early breast cancer: results from the BCIRG 006 Study
Abstract
Background: This study aims to describe and compare health-related quality of life (HRQL) in patients with node-positive and high-risk node-negative HER2-positive early breast cancer receiving adjuvant docetaxel and trastuzumab-based or docetaxel-based regimens alone.
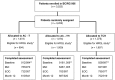
Methods: Eligible patients (n = 3,222) were randomly assigned to either four cycles of adjuvant doxorubicin and cyclophosphamide followed by four cycles of docetaxel (AC→T) or one of two trastuzumab-containing regimens: adjuvant doxorubicin and cyclophosphamide followed by docetaxel plus trastuzumab administered for 1 year (AC→TH) or six cycles of docetaxel plus carboplatin combined with trastuzumab administered for 1 year (TCH). The European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 and BR-23 were administered at baseline, the start of cycle 4 (mid), and the end of chemotherapy (EOC), as well as at 6, 12, and 24 months after chemotherapy.
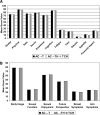
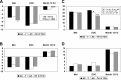
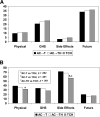
Results: Compliance rates for the EORTC questionnaires were acceptable at 72%-93% of eligible patients out to the 12-month assessment. Systemic side effect (SE) change scores were significantly improved for TCH-treated patients compared with AC→TH and AC→T at EOC, suggesting improved tolerability. Physical functioning (PF) was only slightly worse at midpoint for those receiving TCH, compared with patients who were just starting on taxane in an AC→TH regimen, but was otherwise similar between arms. All treatment arms recovered from the deterioration in SE, PF, and Global Health Scale scores by 1 year and median future perspective change scores continued to improve throughout treatment and follow-up.
Conclusion: HRQL outcomes for adjuvant docetaxel and trastuzumab-based regimens are favorable and support TCH as a more tolerable treatment option.
Keywords: Adjuvant therapy; Anthracycline-induced cardiotoxicity; BCIRG 006; Breast cancer; Chemotherapy; Docetaxel; Quality of life; Trastuzumab.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- National Cancer Institute. Breast cancer. 2011. [Accessed October 14, 2011]. Available at http://www.cancer.gov/cancertopics/types/breast/
-
- Nabholtz JM, Slamon D. New adjuvant strategies for breast cancer: Meeting the challenge of integrating chemotherapy and trastuzumab (Herceptin) Sem Oncol. 2001;28:1–12. - PubMed
-
- Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. - PubMed
-
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. New Eng J Med. 2005;353:1673–1684. - PubMed
-
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. New Eng J Med. 2006;354:809–820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

