Imaging studies in focal dystonias: a systems level approach to studying a systems level disorder
- PMID: 23814533
- PMCID: PMC3580788
- DOI: 10.2174/157015913804999513
Imaging studies in focal dystonias: a systems level approach to studying a systems level disorder
Abstract
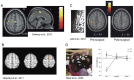
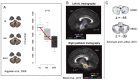
Focal dystonias are dystonias that affect one part of the body, and are sometimes task-specific. Brain imaging and transcranial magnetic stimulation techniques have been valuable in defining the pathophysiology of dystonias in general, and are particularly amenable to studying focal dystonias. Over the past few years, several common themes have emerged in the imaging literature, and this review summarizes these findings and suggests some ways in which these distinct themes might all point to one common systems-level mechanism for dystonia. These themes include (1) the role of premotor regions in focal dystonia, (2) the role of the sensory system and sensorimotor integration in focal dystonia, (3) the role of decreased inhibition/increased excitation in focal dystonia, and (4) the role of brain imaging in evaluating and guiding treatment of focal dystonias. The data across these themes, together with the features of dystonia itself, are consistent with a hypothesis that all dystonias reflect excessive output of postural control/stabilization systems in the brain, and that the mechanisms for dystonia reflect amplification of an existing functional system, rather than recruitment of the wrong motor programs. Imaging is currently being used to test treatment effectiveness, and to visually guide treatment of dystonia, such as placement of deep brain stimulation electrodes. In the future, it is hoped that imaging may be used to individualize treatments across behavioral, pharmacologic, and surgical domains, thus optimizing both the speed and effectiveness of treatment for any given individual with focal dystonia.
Keywords: DBS.; DTI; Dystonia; MEG; PET; TMS; basal ganglia; botulinum toxin; cerebellum; fMRI; posture; premotor.
Figures



References
-
- Defazio G. The epidemiology of primary dystonia: current evidence and perspectives. Eur. J. Neurol. 2010;17( Suppl 1 ):9–14. - PubMed
-
- Bressman SB. Dystonia genotypes, phenotypes, and classification. Adv. Neurol. 2004;94:101–107. - PubMed
-
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121(Pt 7 ):1195–1212. - PubMed
-
- Chen R, Hallett M. Focal dystonia and repetitive motion disorders. Clin. Orthop. Relat. Res. 1998;351 :102–106. - PubMed
-
- Eidelberg D. Functional brain networks in movement disorders. Curr. Opin. Neurol. 1998;11(4 ):319–326. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
