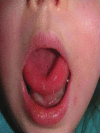
Picibanil (OK-432) in the treatment of head and neck lymphangiomas in children
- PMID: 23814582
- PMCID: PMC3692172
- DOI: 10.4103/1735-3327.109752
Picibanil (OK-432) in the treatment of head and neck lymphangiomas in children
Abstract
Background: Picibanil (OK-432) is a lyophilized mixture of group A Streptococcus pyogenes with antineoplastic activity. Because of its capacity to produce a selective fibrosis of lymphangiomas (LMs), it has been approved by Japanese administration in 1995 for the treatment of LMs.
Materials and methods: We treated 15 children (age range: 6-60 months) affected by head and neck macrocystic LMs with intracystic injections (single dose of 0.2 mL) of Picibanil (1-3 injections).
Results: Complete disappearance of the lesion was noticed in eight (53.33%) cases, a marked (>50%) reduction of LMs was found five (33.33%) cases, while a moderate (<50%) response was recorded in two (13.33%) cases. Picibanil side effects included fever, local inflammation, and transitory increase of blood platelets' concentration; a single case of anemia was resolved with concentrated red blood cells transfusion.
Conclusions: Intracystic injection of Picibanil is an effective and safe treatment for macrocystic LMs in pediatric patients and may represent the treatment of choice in such cases, especially where surgical excision is associated with the risk of functional/cosmetic side effects.
Keywords: Children; lymphangiomas; pediatric age; picibanil (OK-432).
Conflict of interest statement
Figures
References
-
- Kennedy TL. Cystic hygroma-lymphangioma: a rare and still unclear entity. Laryngoscope. 1989;99:1–10. - PubMed
-
- Kennedy TL, Whitaker M, Pellitteri P, Wood WE. Cystic hygroma/lymphangioma: A rational approach to management. Laryngoscope. 2001;111:1929–37. - PubMed
-
- Ogita S, Tsuto T, Tokiwa K, Takahashi T. Intracystic injection of OK-432: A new sclerosing therapy for cystic hygroma in children. Br J Surg. 1987;74:690–1. - PubMed
-
- Oosthuizen JC, Burns P, Russell JD. Lymphatic malformations: A proposed management algorithm. Int J Pediatr Otorhinolaryngol. 2010;74:398–403. - PubMed
-
- Poldervaart MT, Breugem CC, Speleman L, Pasmans S. Treatment of lymphatic malformations with OK-432 (Picibanil): Review of the literature. J Craniofac Surg. 2009;20:1159–62. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources