LINX(®) Reflux Management System in chronic gastroesophageal reflux: a novel effective technology for restoring the natural barrier to reflux
- PMID: 23814607
- PMCID: PMC3667475
- DOI: 10.1177/1756283X13486311
LINX(®) Reflux Management System in chronic gastroesophageal reflux: a novel effective technology for restoring the natural barrier to reflux
Abstract
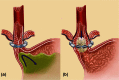
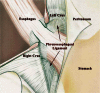
Gastroesophageal reflux disease (GERD) results from incompetency of the lower esophageal sphincter that allows the contents of the stomach to reflux into the esophagus, the airways, and the mouth. The disease affects about 10% of the western population and has a profound negative impact on quality of life. The majority of patients are successfully treated with proton-pump inhibitors, but up to 40% have incomplete relief of symptoms even after dose adjustment. The laparoscopic Nissen fundoplication represents the surgical gold standard, but is largely underused because of the level of technical difficulty and the prevalence of side effects. These factors have contributed to the propensity of patients to continue with medical therapy despite inadequate symptom control and complications of the disease. As a consequence, a significant 'therapy gap' in the treatment of GERD remains evident in current clinical practice. The LINX(®) Reflux Management System (Torax Medical, St. Paul, MN, USA) is designed to provide a permanent solution to GERD by augmenting the sphincter barrier with a standardized, reproducible laparoscopic procedure that does not alter gastric anatomy and is easily reversible. Two single-group trials confirmed that a magnetic device designed to augment the lower esophageal sphincter can be safely and effectively implanted using a standard laparoscopic approach. The device decreased esophageal acid exposure, improved reflux symptoms and quality of life, and allowed cessation of proton-pump inhibitors in the majority of patients.
Keywords: Barrett’s; Nissen; adenocarcinoma; esophagitis; fundoplication; gastroesophageal reflux disease; heartburn; lower esophageal sphincter augmentation; proton-pump inhibitors; regurgitation.
Conflict of interest statement
Figures





References
-
- Becher A., El-Serag H. (2011) Systematic review: the association between symptomatic response to proton pump inhibitors and health-related quality of life in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 34: 618–627 - PubMed
-
- Bonavina L. (2012) Assessment of surgical innovation. Innovation in Esophageal Surgery. Berlin: Springer
-
- Bonavina L. (2013) Selected commentary to “Esophageal sphincter device for gastroesophageal reflux disease”. Eur Surg. DOI: 10.1007/s10353-013-0206-z - DOI
-
- Bonavina L., DeMeester T., Fockens P., Dunn D., Saino G., Bona D., et al. (2010) Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg 252: 857–862 - PubMed
-
- Bonavina L., Saino G., Bona D., Lipham J., Ganz R., Dunn D., et al. (2008) Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg 12: 2133–2140 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

