Rhinovirus-Infected Epithelial Cells Produce More IL-8 and RANTES Compared With Other Respiratory Viruses
- PMID: 23814675
- PMCID: PMC3695236
- DOI: 10.4168/aair.2013.5.4.216
Rhinovirus-Infected Epithelial Cells Produce More IL-8 and RANTES Compared With Other Respiratory Viruses
Abstract
Purpose: The environmental factors human rhinoviruses (HRVs) and house dust mites (HDMs) are the most common causes of acute exacerbations of asthma. The aim of this study was to compare the chemokine production induced by HRVs in airway epithelial cells with that induced by other respiratory viruses, and to investigate synergistic interactions between HRVs and HDMs on the induction of inflammatory chemokines in vitro.
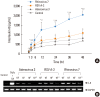
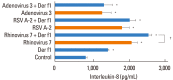
Methods: A549 human airway epithelial cells were infected with either rhinovirus serotype 7, respiratory syncytial virus (RSV)-A2 strain, or adenovirus serotype 3 and analyzed for interleukin (IL)-8 and regulated on activation, normal T-cell expressed and secreted (RANTES) release and mRNA expression. Additionally, activation of nuclear factor (NF)-κB and activator protein (AP)-1 were evaluated. The release of IL-8 and RANTES was also measured in cells stimulated simultaneously with a virus and the HDM allergen, Der f1.
Results: HRV caused greater IL-8 and RANTES release and mRNA expression compared with either RSV or adenovirus. NF-κB and AP-1 were activated in these processes. Cells incubated with a virus and Der f1 showed an increased IL-8 release. However, compared with cells incubated with virus alone as the stimulator, only HRV with Der f1 showed a statistically significant increase.
Conclusions: IL-8 and RANTES were induced to a greater extent by HRV compared with other viruses, and only HRV with Der f1 acted synergistically to induce bronchial epithelial IL-8 release. These findings may correspond with the fact that rhinoviruses are identified more frequently than other viruses in cases of acute exacerbation of asthma.
Keywords: Der f1; IL-8; Rhinovirus; asthma; regulated on activation, normal T-cell expressed and secreted.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

