Keratinocyte growth factor and glucocorticoid induction of human peroxiredoxin 6 gene expression occur by independent mechanisms that are synergistic
- PMID: 23815338
- PMCID: PMC3894679
- DOI: 10.1089/ars.2012.4634
Keratinocyte growth factor and glucocorticoid induction of human peroxiredoxin 6 gene expression occur by independent mechanisms that are synergistic
Abstract
Aims: Peroxiredoxin 6 (Prdx6), a 1-cys Prdx has both peroxidase and phospholipase A2 activities, protecting against oxidative stress and regulating pulmonary surfactant phospholipid metabolism. This study determined the mechanism by which keratinocyte growth factor (KGF) and the glucocorticoid analogue, dexamethasone (Dex), induce increased Prdx6 expression.
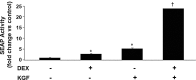
Results: Transcriptional activation by KGF in both A549 lung adenocarcinoma cells and rat lung alveolar epithelial type II (ATII) cells utilizes an antioxidant response element (ARE), located between 357 and 349 nucleotides before the PRDX6 translational start, that is also necessary for upregulation of the human PRDX6 promoter in response to oxidative stress. Activation is mediated by binding of the transcription factor, Nrf2, to the ARE as shown by experiments using siRNA against Nrf2 and by transfecting ATII cells isolated from lungs of Nrf2 null mice. KGF triggers the migration of Nrf2 from cytoplasm to nucleus where it binds to the PRDX6 promoter as shown by chromatin immunoprecipitation assays. Activation of transcription by Dex occurs through a glucocorticoid response element located about 750 nucleotides upstream of the PRDX6 translational start.
Innovation: This study demonstrates that KGF can activate an ARE in a promoter without reactive oxygen species involvement and that KGF and Dex can synergistically activate the PRDX6 promoter and protect cells from oxidative stress.
Conclusion: These two different activators work through different DNA elements. Their combined effect on transcription of the reporter gene is synergistic; however, at the protein level, the combined effect is additive and protects cells from oxidative damage.
Figures











References
-
- Bao S, Wang Y, Sweeney P, Chaudhuri A, Doseff AI, Marsh CB, and Knoell DL. Keratinocyte growth factor induces Akt kinase activity and inhibits Fas-mediated apoptosis in A549 lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 288: L36–L42, 2005 - PubMed
-
- Bortnick AE, Favari E, Tao JQ, Francone OL, Reilly M, Zhang Y, Rothblat GH, and Bates SR. Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 285: L869–L878, 2003 - PubMed
-
- Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, and Werner S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol 22: 5492–5505, 2002 - PMC - PubMed
-
- Chen JW, Dodia C, Feinstein SI, Jain MK, and Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem 275: 28421–28427, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

