Chronic glucolipotoxic conditions in pancreatic islets impair insulin secretion due to dysregulated calcium dynamics, glucose responsiveness and mitochondrial activity
- PMID: 23815372
- PMCID: PMC3704974
- DOI: 10.1186/1471-2121-14-31
Chronic glucolipotoxic conditions in pancreatic islets impair insulin secretion due to dysregulated calcium dynamics, glucose responsiveness and mitochondrial activity
Abstract
Background: In the progression towards diabetes, glucolipotoxicity is one of the main causes of pancreatic beta cell pathology. The aim of this study was to examine the in vitro effects of chronic glucolipotoxic conditions on cellular responses in pancreatic islets, including glucose and fat metabolism, Calcium mobilization, insulin secretion and insulin content.
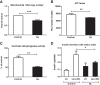
Results: Exposure of islets to chronic glucolipotoxic conditions decreased glucose stimulated insulin secretion in vitro. Reduced protein levels of Glut2/slc2a2, and decreased glucokinase and pyruvate carboxylase mRNA levels indicated a significant lowering in glucose sensing. Concomitantly, both fatty acid uptake and triglyceride accumulation increased significantly while fatty acid oxidation decreased. This general suppression in glucose metabolism correlated well with a decrease in mitochondrial number and activity, reduction in cellular ATP content and dampening of the TCA cycle. Further, we also observed a decrease in IP3 levels and lower Calcium mobilization in response to glucose. Importantly, chronic glucolipotoxic conditions in vitro decreased insulin gene expression, insulin content, insulin granule docking (to the plasma membrane) and insulin secretion.
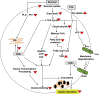
Conclusions: Our results present an integrated view of the effects of chronic glucolipotoxic conditions on known and novel signaling events, in vitro, that results in reduced glucose responsiveness and insulin secretion.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

