Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury
- PMID: 23815563
- PMCID: PMC3814815
- DOI: 10.1089/neu.2013.2970
Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury
Abstract
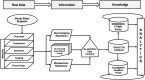
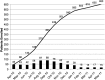
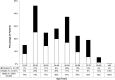
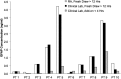
Traumatic brain injury (TBI) is among the leading causes of death and disability worldwide, with enormous negative social and economic impacts. The heterogeneity of TBI combined with the lack of precise outcome measures have been central to the discouraging results from clinical trials. Current approaches to the characterization of disease severity and outcome have not changed in more than three decades. This prospective multicenter observational pilot study aimed to validate the feasibility of implementing the TBI Common Data Elements (TBI-CDEs). A total of 650 subjects who underwent computed tomography (CT) scans in the emergency department within 24 h of injury were enrolled at three level I trauma centers and one rehabilitation center. The TBI-CDE components collected included: 1) demographic, social and clinical data; 2) biospecimens from blood drawn for genetic and proteomic biomarker analyses; 3) neuroimaging studies at 2 weeks using 3T magnetic resonance imaging (MRI); and 4) outcome assessments at 3 and 6 months. We describe how the infrastructure was established for building data repositories for clinical data, plasma biomarkers, genetics, neuroimaging, and multidimensional outcome measures to create a high quality and accessible information commons for TBI research. Risk factors for poor follow-up, TBI-CDE limitations, and implementation strategies are described. Having demonstrated the feasibility of implementing the TBI-CDEs through successful recruitment and multidimensional data collection, we aim to expand to additional study sites. Furthermore, interested researchers will be provided early access to the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) data set for collaborative opportunities to more precisely characterize TBI and improve the design of future clinical treatment trials. (ClinicalTrials.gov Identifier NCT01565551.).
Figures



References
-
- Faul M. Xu L. Wald M.M. Coronado V.G. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta: 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006.
-
- Murray C.J. Lopez A.D. Alternative projections of mortality and disability by cause of disease study. Lancet. 1997;349:1498–504. - PubMed
-
- Bazarian J.J. Veazie P. Mookerjee S. Lerner E.B. Accuracy of mild traumatic brain injury case ascertainment using ICD-9 codes. Acad. Emerg. Med. 2006;13:31–38. - PubMed
-
- Powell J.M. Ferraro J.V. Dikmen S.S. Temkin N.R. Bell K.R. Accuracy of mild traumatic brain injury diagnosis. Arch. Phys. Med. Rehabil. 2008;89:1550–1555. - PubMed
-
- Cancelliere C. Cassidy J.D. Cote P. Hincapie C.A. Hartvigsen J. Carroll L.J. Marras C. Boyle E. Kristman V. Hung R. Stalnacke B.M. Rumney P. Coronado V. Holm L.W. Borg J. Nygren-de Boussard C. Af Geijerstam J.L. Keightley M. Protocol for a systematic review of prognosis after mild traumatic brain injury: an update of the WHO Collaborating Centre Task Force findings. Syst. Rev. 2012;1:17. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

