Lysosome-mediated processing of chromatin in senescence
- PMID: 23816621
- PMCID: PMC3704985
- DOI: 10.1083/jcb.201212110
Lysosome-mediated processing of chromatin in senescence
Abstract
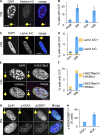
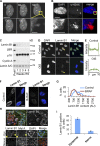
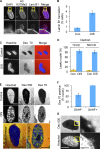
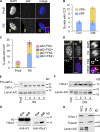
Cellular senescence is a stable proliferation arrest, a potent tumor suppressor mechanism, and a likely contributor to tissue aging. Cellular senescence involves extensive cellular remodeling, including of chromatin structure. Autophagy and lysosomes are important for recycling of cellular constituents and cell remodeling. Here we show that an autophagy/lysosomal pathway processes chromatin in senescent cells. In senescent cells, lamin A/C-negative, but strongly γ-H2AX-positive and H3K27me3-positive, cytoplasmic chromatin fragments (CCFs) budded off nuclei, and this was associated with lamin B1 down-regulation and the loss of nuclear envelope integrity. In the cytoplasm, CCFs were targeted by the autophagy machinery. Senescent cells exhibited markers of lysosomal-mediated proteolytic processing of histones and were progressively depleted of total histone content in a lysosome-dependent manner. In vivo, depletion of histones correlated with nevus maturation, an established histopathologic parameter associated with proliferation arrest and clinical benignancy. We conclude that senescent cells process their chromatin via an autophagy/lysosomal pathway and that this might contribute to stability of senescence and tumor suppression.
Figures


Comment in
-
Probing the depths of cellular senescence.J Cell Biol. 2013 Jul 8;202(1):11-3. doi: 10.1083/jcb.201305155. Epub 2013 Jul 1. J Cell Biol. 2013. PMID: 23816622 Free PMC article.
References
-
- Barnhill R., Piepkorn M., Busam K., 2004. Pathology of Melanocytic Nevi and Malignant Melanoma. Springer; 2nd ed, XIV, 406 pp
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

