Proteolytically activated anti-bacterial hydrogel microspheres
- PMID: 23816641
- PMCID: PMC3795988
- DOI: 10.1016/j.jconrel.2013.06.023
Proteolytically activated anti-bacterial hydrogel microspheres
Abstract
Hydrogels are finding increased clinical utility as advances continue to exploit their favorable material properties. Hydrogels can be adapted for many applications, including surface coatings and drug delivery. Anti-infectious surfaces and delivery systems that actively destroy invading organisms are alternative ways to exploit the favorable material properties offered by hydrogels. Sterilization techniques are commonly employed to ensure the materials are non-infectious upon placement, but sterilization is not absolute and infections are still expected. Natural, anti-bacterial proteins have been discovered which have the potential to act as anti-infectious agents; however, the proteins are toxic and need localized release to have therapeutic efficacy without toxicity. In these studies, we explore the use of the glutathione s-transferase (GST) to anchor the bactericidal peptide, melittin, to the surface of poly(ethylene glycol) diacrylate (PEGDA) hydrogel microspheres. We show that therapeutic levels of protein can be anchored to the surface of the microspheres using the GST anchor. We compared the therapeutic efficacy of recombinant melittin released from PEGDA microspheres to melittin. We found that, when released by an activating enzyme, thrombin, recombinant melittin efficiently inhibits growth of the pathogenic bacterium Streptococcus pyogenes as effectively as melittin created by solid phase peptide synthesis. We conclude that a GST protein anchor can be used to immobilize functional protein to PEGDA microspheres and the protein will remain immobilized under physiological conditions until the protein is enzymatically released.
Keywords: Glutathione; Glutathione s-transferase; Hydrogel; Microparticles; Recombinant protein; Thrombin.
© 2013.
Figures


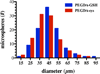
 ), 1 mM (
), 1 mM ( ), 0.1 mM (
), 0.1 mM ( ), 0.01 mM (
), 0.01 mM ( ), and 0 mM (
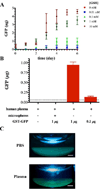
), and 0 mM ( ) GSH containing phosphate buffered saline. Each point represents the mean plus or minus the standard deviation of three independent samples. (B) Human plasma mediated release of GFP from GST-GFP loaded PEGDA-GSH microspheres. Microspheres were incubated with human plasma for 6 days at 37°C prior to measuring GFP in the supernatant by fluorescence. Each point represents the mean plus or minus the standard error of the mean of three independent samples in plasma from 3 human donors. (C) Gross photographs of microspheres under UV illumination after 6 days of incubation in human plasma or PBS at 37°C. The scale bar is 1 mm.
) GSH containing phosphate buffered saline. Each point represents the mean plus or minus the standard deviation of three independent samples. (B) Human plasma mediated release of GFP from GST-GFP loaded PEGDA-GSH microspheres. Microspheres were incubated with human plasma for 6 days at 37°C prior to measuring GFP in the supernatant by fluorescence. Each point represents the mean plus or minus the standard error of the mean of three independent samples in plasma from 3 human donors. (C) Gross photographs of microspheres under UV illumination after 6 days of incubation in human plasma or PBS at 37°C. The scale bar is 1 mm.
 ) 0, 11 (
) 0, 11 ( ), 22 (
), 22 ( ), 44 (
), 44 ( ), or 110 (
), or 110 ( ) nM thrombin as detected by fluorescent GFP signal. Each point represents the mean plus or minus the standard deviation of three independent samples. (B) Representative brightfield and epifluorescent micrographs depicting GST-GFP (green) associated with microspheres before (top row) or after (bottom row) release in response to thrombin (1.8 µM). (C) Rate (v) of GFP liberation from GST-GFP (unbound;
) nM thrombin as detected by fluorescent GFP signal. Each point represents the mean plus or minus the standard deviation of three independent samples. (B) Representative brightfield and epifluorescent micrographs depicting GST-GFP (green) associated with microspheres before (top row) or after (bottom row) release in response to thrombin (1.8 µM). (C) Rate (v) of GFP liberation from GST-GFP (unbound;  ) and GST-GFP (bound;
) and GST-GFP (bound;  ) bound to PEGDA-GSH microspheres for the linear portion of the rate curve, typically the first 1 hour. Each point represents the mean plus or minus the standard deviation of three independent samples.
) bound to PEGDA-GSH microspheres for the linear portion of the rate curve, typically the first 1 hour. Each point represents the mean plus or minus the standard deviation of three independent samples.
Similar articles
-
PEG-stabilized lipid disks as carriers for amphiphilic antimicrobial peptides.J Control Release. 2011 Dec 20;156(3):323-8. doi: 10.1016/j.jconrel.2011.08.029. Epub 2011 Aug 28. J Control Release. 2011. PMID: 21903146
-
Multiplex immunoassay platforms based on shape-coded poly(ethylene glycol) hydrogel microparticles incorporating acrylic acid.Sensors (Basel). 2012;12(6):8426-36. doi: 10.3390/s120608426. Epub 2012 Jun 20. Sensors (Basel). 2012. PMID: 22969408 Free PMC article.
-
A comparative study of polyethylene glycol hydrogels derivatized with the RGD peptide and the cell-binding domain of fibronectin.J Biomed Mater Res A. 2014 Jan;102(1):170-9. doi: 10.1002/jbm.a.34687. Epub 2013 Apr 24. J Biomed Mater Res A. 2014. PMID: 23613303
-
Liquid-liquid two-phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical applications: A tutorial review.Acta Biomater. 2011 Jan;7(1):31-56. doi: 10.1016/j.actbio.2010.07.028. Epub 2010 Jul 24. Acta Biomater. 2011. PMID: 20659596 Free PMC article. Review.
-
Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications.J Control Release. 2014 Nov 10;193:324-40. doi: 10.1016/j.jconrel.2014.09.003. Epub 2014 Sep 10. J Control Release. 2014. PMID: 25218676 Review.
Cited by
-
Zinc-triggered hydrogelation of self-assembled small molecules to inhibit bacterial growth.Sci Rep. 2015 Jan 13;5:7753. doi: 10.1038/srep07753. Sci Rep. 2015. PMID: 25583430 Free PMC article.
-
Research progress on blood compatibility of hemoperfusion adsorbent materials.Front Bioeng Biotechnol. 2024 Oct 1;12:1456694. doi: 10.3389/fbioe.2024.1456694. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39411060 Free PMC article. Review.
-
Melittin-glutathione S-transferase fusion protein exhibits anti-inflammatory properties and minimal toxicity.Eur J Pharm Sci. 2014 Dec 18;65:112-21. doi: 10.1016/j.ejps.2014.09.012. Epub 2014 Sep 21. Eur J Pharm Sci. 2014. PMID: 25240321 Free PMC article.
-
Assessment of antibiotic release and antibacterial efficacy from pendant glutathione hydrogels using ex vivo porcine skin.J Control Release. 2024 Jan;365:936-949. doi: 10.1016/j.jconrel.2023.12.008. Epub 2023 Dec 19. J Control Release. 2024. PMID: 38070603 Free PMC article.
-
Glutathione-Conjugated Hydrogels: Flexible Vehicles for Personalized Treatment of Bacterial Infections.Pharm Res. 2021 Jul;38(7):1247-1261. doi: 10.1007/s11095-021-03057-1. Epub 2021 Jun 11. Pharm Res. 2021. PMID: 34117588 Free PMC article.
References
-
- Haley RW, Hooton TM, et al. Nosocomial infections in U.S. hospitals, 1975–1976: estimated frequency by selected characteristics of patients. Am J Med. 1981;70:947–959. - PubMed
-
- Wisplinghoff H, Bischoff T, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. - PubMed
-
- D'Este M, Eglin D. Hydrogels in calcium phosphate moldable and injectable bone substitutes: Sticky excipients or advanced 3-D carriers? Acta Biomater. 2013;9:5421–5430. - PubMed
-
- Nablo BJ, Schoenfisch MH. Antibacterial properties of nitric oxide-releasing sol-gels. J Biomed Mater Res A. 2003;67:1276–1283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

