Phase II study of topotecan and bevacizumab in advanced, refractory non--small-cell lung cancer
- PMID: 23816875
- PMCID: PMC4524809
- DOI: 10.1016/j.cllc.2013.04.009
Phase II study of topotecan and bevacizumab in advanced, refractory non--small-cell lung cancer
Abstract
Background: This clinical trial evaluated whether topotecan in combination with bevacizumab improved progression-free survival (PFS) in patients with advanced, refractory non--small-cell lung cancer in a second-line setting.
Patient and methods: Patients aged 18 years old and older received topotecan (4.0 mg/m(2)) on days 1, 8, and 15, and bevacizumab (10 mg/kg) on days 1 and 15 as intravenous infusions on a 28-day treatment cycle. Available tumor specimens were analyzed for ISG15 gene expression as a biomarker of response to topotecan.
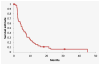
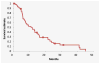
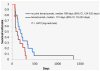
Results: Forty-two patients were enrolled in the study, with a median age of 62.5 years and a median of 3 (range, 1-7) prior treatment regimens. Almost half (n = 18, 42.9%) of the patients received prior bevacizumab therapy. PFS was 5.1 months (95% CI, 3.7-7.8 months), and overall survival was 11.5 months (95% CI, 6.8-15.5 months). Response rates were as follows: 14.3% partial response, 54.8% stable disease, and 28.6% progressive disease. Hematologic toxicities included grade 3 thrombocytopenia (n = 7, 16.7%), neutropenia (n = 4, 9.5%), and anemia (n = 2, 4.8%). One toxic death occurred due to pulmonary hemorrhage, and one patient experienced a grade 4 pulmonary embolism. Grade 3 nonhematologic adverse events were uncommon (< 8%). There was a trend for improved median PFS, 3.5 months vs. 1.8 months (P = .26), in patients with high ISG15 expression.
Conclusion: Bevacizumab in combination with topotecan as a salvage therapy for metastatic non--small-cell lung cancer is well tolerated and is worthy of further investigation.
Keywords: Bevacizumab; ISG15 expression; Non-small-cell lung cancer; Refractory; Second-line therapy; Topotecan.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. - PubMed
-
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. - PubMed
-
- Shepherd FA, Pereira JR, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;252:123–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous