A single intravenous rAAV injection as late as P20 achieves efficacious and sustained CNS Gene therapy in Canavan mice
- PMID: 23817205
- PMCID: PMC3863789
- DOI: 10.1038/mt.2013.138
A single intravenous rAAV injection as late as P20 achieves efficacious and sustained CNS Gene therapy in Canavan mice
Abstract
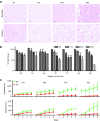
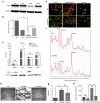
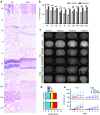
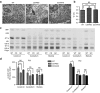
Canavan's disease (CD) is a fatal pediatric leukodystrophy caused by mutations in aspartoacylase (AspA) gene. Currently, there is no effective treatment for CD; however, gene therapy is an attractive approach to ameliorate the disease. Here, we studied progressive neuropathology and gene therapy in short-lived (≤ 1 month) AspA(-/-) mice, a bona-fide animal model for the severest form of CD. Single intravenous (IV) injections of several primate-derived recombinant adeno-associated viruses (rAAVs) as late as postnatal day 20 (P20) completely rescued their early lethality and alleviated the major disease symptoms, extending survival in P0-injected rAAV9 and rAAVrh8 groups to as long as 2 years thus far. We successfully used microRNA (miRNA)-mediated post-transcriptional detargeting for the first time to restrict therapeutic rAAV expression in the central nervous system (CNS) and minimize potentially deleterious effects of transgene overexpression in peripheral tissues. rAAV treatment globally improved CNS myelination, although some abnormalities persisted in the content and distribution of myelin-specific and -enriched lipids. We demonstrate that systemically delivered and CNS-restricted rAAVs can serve as efficacious and sustained gene therapeutics in a model of a severe neurodegenerative disorder even when administered as late as P20.
Figures


References
-
- Van Bogaert L. Sur une idiotie familiale avec dégénérescence spongieuse de nevraxe. Acta Neurol Belg. 1949;49:572–587.
-
- Traeger EC, Rapin I. The clinical course of Canavan disease. Pediatr Neurol. 1998;18:207–212. - PubMed
-
- Matalon RM, Michals-Matalon K. Spongy degeneration of the brain, Canavan disease: biochemical and molecular findings. Front Biosci. 2000;5:D307–D311. - PubMed
-
- Beaudet A.2001Aspartoacylase deficiency (Canavan disease) Scriver CR, Beaudet AL, Sly WS, Valle D.eds). The Metabolic and Molecular Bases of Inherited Disease pp. 5799–5805.
-
- Matalon R, Michals K, Sebesta D, Deanching M, Gashkoff P, Casanova J. Aspartoacylase deficiency and N-acetylaspartic aciduria in patients with Canavan disease. Am J Med Genet. 1988;29:463–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

