Drug-drug interactions during antiviral therapy for chronic hepatitis C
- PMID: 23817323
- PMCID: PMC4634866
- DOI: 10.1038/nrgastro.2013.106
Drug-drug interactions during antiviral therapy for chronic hepatitis C
Abstract
The emergence of direct-acting antiviral agents (DAAs) for HCV infection represents a major advance in treatment. The NS3 protease inhibitors, boceprevir and telaprevir, were the first DAAs to receive regulatory approval. When combined with PEG-IFN and ribavirin, these agents increase rates of sustained virologic response in HCV genotype 1 to ∼70%. However, this treatment regimen is associated with several toxicities. In addition, both boceprevir and telaprevir are substrates for and inhibitors of the drug transporter P-glycoprotein and the cytochrome P450 enzyme 3A4 and are, therefore, prone to clinically relevant drug interactions. Several new DAAs for HCV are in late stages of clinical development and are likely to be approved in the near future. These include the protease inhibitors, simeprevir and faldaprevir, the NS5A inhibitor, daclatasvir, and the nucleotide polymerase inhibitor, sofosbuvir. Herein, we review the clinical pharmacology and drug interactions of boceprevir, telaprevir and these investigational DAAs. Although boceprevir and telaprevir are involved in many interactions, these interactions are manageable if health-care providers proactively identify and adjust treatments. Emerging DAAs seem to have a reduced potential for drug interactions, which will facilitate their use in the treatment of HCV.
Figures
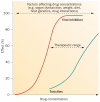

References
-
- WHO. Hepatitis C Fact Sheet. WHO [online] 2013 http://www.who.int/mediacentre/factsheets/fs164/en/
-
- Tang H, Grise H. Cellular and molecular biology of HCV infection and hepatitis. Clin. Sci. (Lond.) 2012;117:49–65. - PubMed
-
- Zein NN, et al. Hepatitis C virus genotypes in the United States: epidemiology, pathogenicity, and response to interferon therapy. Collaborative Study Group. Ann. Intern. Med. 1996;125:634–639. - PubMed
-
- Jacobson IM, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 2011;364:2405–2416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

