Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake
- PMID: 23817545
- PMCID: PMC3939030
- DOI: 10.1038/nn.3445
Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake
Abstract
Compulsive drinking despite serious adverse medical, social and economic consequences is a characteristic of alcohol use disorders in humans. Although frontal cortical areas have been implicated in alcohol use disorders, little is known about the molecular mechanisms and pathways that sustain aversion-resistant intake. Here, we show that nucleus accumbens core (NAcore) NMDA-type glutamate receptors and medial prefrontal (mPFC) and insula glutamatergic inputs to the NAcore are necessary for aversion-resistant alcohol consumption in rats. Aversion-resistant intake was associated with a new type of NMDA receptor adaptation, in which hyperpolarization-active NMDA receptors were present at mPFC and insula but not amygdalar inputs in the NAcore. Accordingly, inhibition of Grin2c NMDA receptor subunits in the NAcore reduced aversion-resistant alcohol intake. None of these manipulations altered intake when alcohol was not paired with an aversive consequence. Our results identify a mechanism by which hyperpolarization-active NMDA receptors under mPFC- and insula-to-NAcore inputs sustain aversion-resistant alcohol intake.
Figures
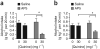
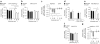
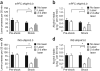

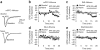
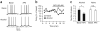
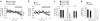
Comment in
-
Drinking through the pain.Nat Neurosci. 2013 Aug;16(8):987-8. doi: 10.1038/nn.3476. Nat Neurosci. 2013. PMID: 23887132 Free PMC article.
References
-
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(suppl. 2):S145–S153. - PubMed
-
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. - PubMed
-
- Wolffgramm J, Galli G, Thimm F, Heyne A. Animal models of addiction: models for therapeutic strategies? J Neural Transm. 2000;107:649–668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

