Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation
- PMID: 23817546
- PMCID: PMC3866024
- DOI: 10.1038/nn.3451
Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation
Abstract
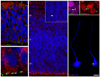
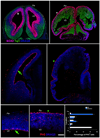
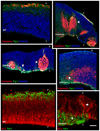
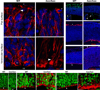
The construction of cerebral cortex begins with the formation of radial glia. Once formed, polarized radial glial cells divide either symmetrically or asymmetrically to balance appropriate production of progenitor cells and neurons. Following birth, neurons use the processes of radial glia as scaffolding for oriented migration. Radial glia therefore provide an instructive structural matrix to coordinate the generation and placement of distinct groups of cortical neurons in the developing cerebral cortex. We found that Arl13b, a cilia-enriched small GTPase that is mutated in Joubert syndrome, was critical for the initial formation of the polarized radial progenitor scaffold. Using developmental stage-specific deletion of Arl13b in mouse cortical progenitors, we found that early neuroepithelial deletion of ciliary Arl13b led to a reversal of the apical-basal polarity of radial progenitors and aberrant neuronal placement. Arl13b modulated ciliary signaling necessary for radial glial polarity. Our findings indicate that Arl13b signaling in primary cilia is crucial for the initial formation of a polarized radial glial scaffold and suggest that disruption of this process may contribute to aberrant neurodevelopment and brain abnormalities in Joubert syndrome-related ciliopathies.
Figures


References
-
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. - PubMed
-
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. - PubMed
-
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. - PubMed
-
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

