The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis
- PMID: 23818109
- PMCID: PMC3974160
- DOI: 10.1002/art.38070
The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis
Abstract
Objective: To study the effect of tumor necrosis factor α (TNFα) inhibitors on progressive spinal damage in patients with ankylosing spondylitis (AS).
Methods: All AS patients meeting the modified New York criteria who had been monitored prospectively and had at least 2 sets of spinal radiographs a minimum of 1.5 years apart were included in the study (n=334). The patients received standard therapy, which included nonsteroidal antiinflammatory drugs and TNFα inhibitors. Radiographic severity was assessed by the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). Patients with a rate of AS progression that was ≥1 mSASSS unit/year were considered progressors. Univariable and multivariable regression analyses were done. Propensity score matching and sensitivity analysis were performed. A zero-inflated negative binomial (ZINB) model was used to analyze the effect of TNFα inhibitors on the change in the mSASSS with varying followup periods. Potential confounders, such as disease activity (as assessed by the Bath Ankylosing Spondylitis Disease Activity Index), the erythrocyte sedimentation rate, C-reactive protein level, HLA-B27 positivity, sex, age at onset, smoking burden (number of pack-years), and baseline damage, were included in the model.
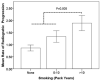
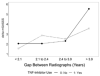
Results: TNFα inhibitor treatment was associated with a 50% reduction in the odds of progression, with an odds ratio (OR) of 0.52 (95% confidence interval [95% CI] 0.30-0.88, P=0.02). Patients with a delay of >10 years in starting therapy were more likely to experience progression as compared to those who started earlier (OR 2.4 [95% CI 1.09-5.3], P=0.03). In the ZINB model, the use of TNFα inhibitors significantly reduced disease progression when the gap between radiographs was >3.9 years. The protective effect of TNFα inhibitors was stronger after propensity score matching.
Conclusion: Treatment with TNFα inhibitors appears to reduce radiographic progression in AS patients, especially with early initiation and with longer duration of followup.
Copyright © 2013 by the American College of Rheumatology.
Figures

Comment in
-
Anti-tumor necrosis factor and new bone formation in ankylosing spondylitis: the controversy continues.Arthritis Rheum. 2013 Oct;65(10):2537-40. doi: 10.1002/art.38068. Arthritis Rheum. 2013. PMID: 23818040 No abstract available.
References
-
- Khan MA, van der Linden SM. Ankylosing spondylitis and other spondyloarthropathies. Rheum Dis Clin North Am. 1990;16(3):551–579. - PubMed
-
- Revicki DA, Luo MP, Wordsworth P, et al. Adalimumab reduces pain, fatigue, and stiffness in patients with ankylosing spondylitis: Results from the adalimumab trial evaluating long-term safety and efficacy for ankylosing spondylitis (ATLAS). J Rheumatol. 2008;35(7):1346–1353. - PubMed
-
- van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: Results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 2005;52(2):582–591. - PubMed
-
- Braun J, van der Horst-Bruinsma IE, Huang F, et al. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: A randomized, double blind trial. Arthritis Rheum. 2011;63(6):1543–1551. - PubMed
-
- Inman RD, Davis JC, Jr, Heijde D, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: Results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58(11):3402–3412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

