Global view of the functional molecular organization of the avian cerebrum: mirror images and functional columns
- PMID: 23818122
- PMCID: PMC4145244
- DOI: 10.1002/cne.23404
Global view of the functional molecular organization of the avian cerebrum: mirror images and functional columns
Abstract
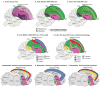
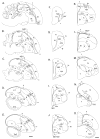
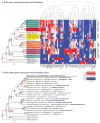
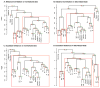
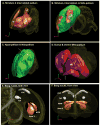
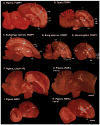
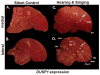
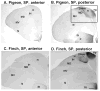
Based on quantitative cluster analyses of 52 constitutively expressed or behaviorally regulated genes in 23 brain regions, we present a global view of telencephalic organization of birds. The patterns of constitutively expressed genes revealed a partial mirror image organization of three major cell populations that wrap above, around, and below the ventricle and adjacent lamina through the mesopallium. The patterns of behaviorally regulated genes revealed functional columns of activation across boundaries of these cell populations, reminiscent of columns through layers of the mammalian cortex. The avian functionally regulated columns were of two types: those above the ventricle and associated mesopallial lamina, formed by our revised dorsal mesopallium, hyperpallium, and intercalated hyperpallium; and those below the ventricle, formed by our revised ventral mesopallium, nidopallium, and intercalated nidopallium. Based on these findings and known connectivity, we propose that the avian pallium has four major cell populations similar to those in mammalian cortex and some parts of the amygdala: 1) a primary sensory input population (intercalated pallium); 2) a secondary intrapallial population (nidopallium/hyperpallium); 3) a tertiary intrapallial population (mesopallium); and 4) a quaternary output population (the arcopallium). Each population contributes portions to columns that control different sensory or motor systems. We suggest that this organization of cell groups forms by expansion of contiguous developmental cell domains that wrap around the lateral ventricle and its extension through the middle of the mesopallium. We believe that the position of the lateral ventricle and its associated mesopallium lamina has resulted in a conceptual barrier to recognizing related cell groups across its border, thereby confounding our understanding of homologies with mammals.
Keywords: amygdala; basal ganglia; brain evolution; brain organization; brain pathways; claustrum; cortex; forebrain; immediate early genes; motor behavior; neural activity; neurotransmitter receptors; pallidum; pallium; primary sensory; striatum.
Copyright © 2013 Wiley Periodicals, Inc.
Figures

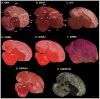
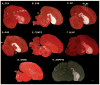

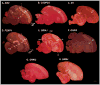
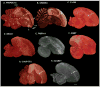
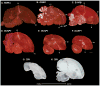
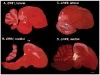
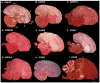
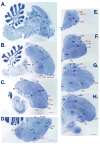








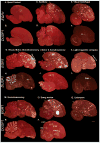
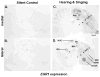
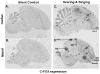


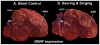

Comment in
-
The impact of gene expression analysis on evolving views of avian brain organization.J Comp Neurol. 2013 Nov;521(16):3604-13. doi: 10.1002/cne.23403. J Comp Neurol. 2013. PMID: 23818089
-
Evolution of the forebrain - revisiting the pallium.J Comp Neurol. 2013 Nov;521(16):3601-3. doi: 10.1002/cne.23444. J Comp Neurol. 2013. PMID: 23893869 No abstract available.
References
-
- Abellan A, Medina L. Subdivisions and derivatives of the chicken subpallium based on expression of LIM and other regulatory genes and markers of neuron sub-populations during development. J Comp Neurol. 2009;515:465–501. - PubMed
-
- Abellan A, Menuet A, Dehay C, Medina L, Retaux S. Differential expression of LIM-homeodomain factors in Cajal-Retzius cells of primates, rodents, and birds. Cereb Cortex. 2009;20:1788–1798. - PubMed
-
- Ariens Kappers CU, Huber CG, Crosby EC. Comparative Anatomy of the Nervous System of Vertebrates, Including Man. New York: Hafner; 1936. Reprinted 1960.
-
- Aboitiz F. Genetic and developmental homology in amniote brains. Toward conciliating radical views of brain evolution. Brain Res Bull. 2011;84:125–136. - PubMed
-
- Atoji Y, Karim MR. Expression of the neocortical marker, RORβ, in the entopallium and field L2 of adult chicken. Neurosci Lett. 2012;521:119–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

