EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype
- PMID: 23818594
- PMCID: PMC3718120
- DOI: 10.1073/pnas.1303058110
EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype
Abstract
The airway epithelium of smokers acquires pathological phenotypes, including basal cell (BC) and/or goblet cell hyperplasia, squamous metaplasia, structural and functional abnormalities of ciliated cells, decreased number of secretoglobin (SCGB1A1)-expressing secretory cells, and a disordered junctional barrier. In this study, we hypothesized that smoking alters airway epithelial structure through modification of BC function via an EGF receptor (EGFR)-mediated mechanism. Analysis of the airway epithelium revealed that EGFR is enriched in airway BCs, whereas its ligand EGF is induced by smoking in ciliated cells. Exposure of BCs to EGF shifted the BC differentiation program toward the squamous and epithelial-mesenchymal transition-like phenotypes with down-regulation of genes related to ciliogenesis, secretory differentiation, and markedly reduced junctional barrier integrity, mimicking the abnormalities present in the airways of smokers in vivo. These data suggest that activation of EGFR in airway BCs by smoking-induced EGF represents a unique mechanism whereby smoking can alter airway epithelial differentiation and barrier function.
Keywords: airway epithelial barrier; cigarette smoking; progenitor cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
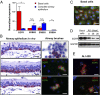
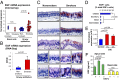
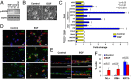
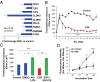
Comment in
-
Basal cell origins of smoking-induced airway epithelial disorders.Cell Cycle. 2014;13(3):341-2. doi: 10.4161/cc.27510. Epub 2013 Dec 13. Cell Cycle. 2014. PMID: 24335435 Free PMC article. No abstract available.
References
-
- Rawlins EL, Hogan BL. Epithelial stem cells of the lung: Privileged few or opportunities for many? Development. 2006;133(13):2455–2465. - PubMed
-
- Hajj R, et al. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells. 2007;25(1):139–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

