Fat cells directly sense temperature to activate thermogenesis
- PMID: 23818608
- PMCID: PMC3725077
- DOI: 10.1073/pnas.1310261110
Fat cells directly sense temperature to activate thermogenesis
Abstract
Classic brown fat and inducible beige fat both dissipate chemical energy in the form of heat through the actions of mitochondrial uncoupling protein 1. This nonshivering thermogenesis is crucial for mammals as a defense against cold and obesity/diabetes. Cold is known to act indirectly through the sympathetic nervous systems and β-adrenergic signaling, but here we report that cool temperature (27-33 °C) can directly activate a thermogenic gene program in adipocytes in a cell-autonomous manner. White and beige fat cells respond to cool temperatures, but classic brown fat cells do not. Importantly, this activation in isolated cells is independent of the canonical cAMP/Protein Kinase A/cAMP response element-binding protein pathway downstream of the β-adrenergic receptors. These findings provide an unusual insight into the role of adipose tissues in thermoregulation, as well as an alternative way to target nonshivering thermogenesis for treatment of obesity and metabolic diseases.
Keywords: Ucp1; cold sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
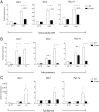
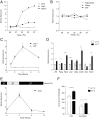
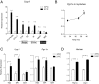
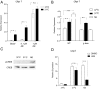
References
-
- Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84(1):277–359. - PubMed
-
- Lowell BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366(6457):740–742. - PubMed
-
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9(2):203–209. - PubMed
-
- van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–1508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

