Posttranslational modification of CENP-A influences the conformation of centromeric chromatin
- PMID: 23818633
- PMCID: PMC3718089
- DOI: 10.1073/pnas.1300325110
Posttranslational modification of CENP-A influences the conformation of centromeric chromatin
Abstract
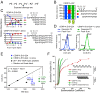
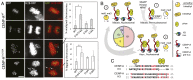
Centromeres are chromosomal loci required for accurate segregation of sister chromatids during mitosis. The location of the centromere on the chromosome is not dependent on DNA sequence, but rather it is epigenetically specified by the histone H3 variant centromere protein A (CENP-A). The N-terminal tail of CENP-A is highly divergent from other H3 variants. Canonical histone N termini are hotspots of conserved posttranslational modification; however, no broadly conserved modifications of the vertebrate CENP-A tail have been previously observed. Here, we report three posttranslational modifications on human CENP-A N termini using high-resolution MS: trimethylation of Gly1 and phosphorylation of Ser16 and Ser18. Our results demonstrate that CENP-A is subjected to constitutive initiating methionine removal, similar to other H3 variants. The nascent N-terminal residue Gly1 becomes trimethylated on the α-amino group. We demonstrate that the N-terminal RCC1 methyltransferase is capable of modifying the CENP-A N terminus. Methylation occurs in the prenucleosomal form and marks the majority of CENP-A nucleosomes. Serine 16 and 18 become phosphorylated in prenucleosomal CENP-A and are phosphorylated on asynchronous and mitotic nucleosomal CENP-A and are important for chromosome segregation during mitosis. The double phosphorylation motif forms a salt-bridged secondary structure and causes CENP-A N-terminal tails to form intramolecular associations. Analytical ultracentrifugation of phospho-mimetic CENP-A nucleosome arrays demonstrates that phosphorylation results in greater intranucleosome associations and counteracts the hyperoligomerized state exhibited by unmodified CENP-A nucleosome arrays. Our studies have revealed that the major modifications on the N-terminal tail of CENP-A alter the physical properties of the chromatin fiber at the centromere.
Keywords: epigenetics; kinetochore; mass spectrometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cleveland DW, Mao Y, Sullivan KF. Centromeres and kinetochores: From epigenetics to mitotic checkpoint signaling. Cell. 2003;112(4):407–421. - PubMed
-
- Foltz DR, et al. The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol. 2006;8(5):458–469. - PubMed
-
- Okada M, et al. The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat Cell Biol. 2006;8(5):446–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

