Enhanced transcription rates in membrane-free protocells formed by coacervation of cell lysate
- PMID: 23818642
- PMCID: PMC3718175
- DOI: 10.1073/pnas.1222321110
Enhanced transcription rates in membrane-free protocells formed by coacervation of cell lysate
Abstract
Liquid-liquid phase transitions in complex mixtures of proteins and other molecules produce crowded compartments supporting in vitro transcription and translation. We developed a method based on picoliter water-in-oil droplets to induce coacervation in Escherichia coli cell lysate and follow gene expression under crowded and noncrowded conditions. Coacervation creates an artificial cell-like environment in which the rate of mRNA production is increased significantly. Fits to the measured transcription rates show a two orders of magnitude larger binding constant between DNA and T7 RNA polymerase, and five to six times larger rate constant for transcription in crowded environments, strikingly similar to in vivo rates. The effect of crowding on interactions and kinetics of the fundamental machinery of gene expression has a direct impact on our understanding of biochemical networks in vivo. Moreover, our results show the intrinsic potential of cellular components to facilitate macromolecular organization into membrane-free compartments by phase separation.
Keywords: macromolecular crowding; microdroplets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

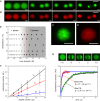
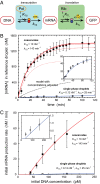

References
-
- Rasmussen S, et al. Evolution. Transitions from nonliving to living matter. Science. 2004;303(5660):963–965. - PubMed
-
- Rasmussen S, Chen LH, Nilsson M, Abe S. Bridging nonliving and living matter. Artif Life. 2003;9(3):269–316. - PubMed
-
- Szostak JW, Bartel DP, Luisi PL. Synthesizing life. Nature. 2001;409(6818):387–390. - PubMed
-
- Hyman AA, Simons K. Cell biology. Beyond oil and water—phase transitions in cells. Science. 2012;337(6098):1047–1049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

