Natural killer cells in HIV controller patients express an activated effector phenotype and do not up-regulate NKp44 on IL-2 stimulation
- PMID: 23818644
- PMCID: PMC3718138
- DOI: 10.1073/pnas.1302090110
Natural killer cells in HIV controller patients express an activated effector phenotype and do not up-regulate NKp44 on IL-2 stimulation
Abstract
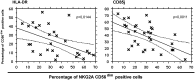
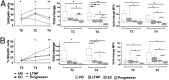
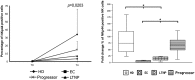
Control of HIV replication in elite controller (EC) and long-term nonprogressor (LTNP) patients has been associated with efficient CD8(+)cytotoxic T-lymphocyte function. However, innate immunity may play a role in HIV control. We studied the expression of natural cytotoxicity receptors (NKp46, NKp30, and NKp44) and their induction over a short time frame (2-4 d) on activation of natural killer (NK) cells in 31 HIV controller patients (15 ECs, 16 LTNPs). In EC/LTNP, induction of NKp46 expression was normal but short (2 d), and NKp30 was induced to lower levels vs. healthy donors. Notably, in antiretroviral-treated aviremic progressor patients (TAPPs), no induction of NKp46 or NKp30 expression occurred. More importantly, EC/LTNP failed to induce expression of NKp44, a receptor efficiently induced in activated NK cells in TAPPs. The specific lack of NKp44 expression resulted in sharply decreased capability of killing target cells by NKp44, whereas TAPPs had conserved NKp44-mediated lysis. Importantly, conserved NK cell responses, accompanied by a selective defect in the NKp44-activating pathway, may result in lack of killing of uninfected CD4(+)NKp44Ligand(+) cells when induced by HIVgp41 peptide-S3, representing a relevant mechanism of CD4(+) depletion. In addition, peripheral NK cells from EC/LTNP had increased NKG2D expression, significant HLA-DR up-regulation, and a mature (NKG2A-CD57(+)killer cell Ig-like receptor(+)CD85j(+)) phenotype, with cytolytic function also against immature dendritic cells. Thus, NK cells in EC/LTNP can maintain substantially unchanged functional capabilities, whereas the lack of NKp44 induction may be related to CD4 maintenance, representing a hallmark of these patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- O’Connell KA, Bailey JR, Blankson JN. Elucidating the elite: Mechanisms of control in HIV-1 infection. Trends Pharmacol Sci. 2009;30(12):631–637. - PubMed
-
- Poropatich K, Sullivan DJ., Jr Human immunodeficiency virus type 1 long-term non-progressors: The viral, genetic and immunological basis for disease non-progression. J Gen Virol. 2011;92(Pt 2):247–268. - PubMed
-
- Lamine A, et al. Replication-competent HIV strains infect HIV controllers despite undetectable viremia (ANRS EP36 study) AIDS. 2007;21(8):1043–1045. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

