Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function
- PMID: 23818689
- PMCID: PMC3784196
- DOI: 10.1113/jphysiol.2013.259382
Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function
Abstract
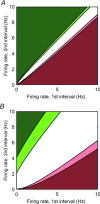
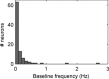
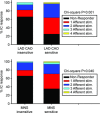
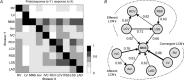
The aims of the study were to determine how aggregates of intrinsic cardiac (IC) neurons transduce the cardiovascular milieu versus responding to changes in central neuronal drive and to determine IC network interactions subsequent to induced neural imbalances in the genesis of atrial fibrillation (AF). Activity from multiple IC neurons in the right atrial ganglionated plexus was recorded in eight anaesthetized canines using a 16-channel linear microelectrode array. Induced changes in IC neuronal activity were evaluated in response to: (1) focal cardiac mechanical distortion; (2) electrical activation of cervical vagi or stellate ganglia; (3) occlusion of the inferior vena cava or thoracic aorta; (4) transient ventricular ischaemia, and (5) neurally induced AF. Low level activity (ranging from 0 to 2.7 Hz) generated by 92 neurons was identified in basal states, activities that displayed functional interconnectivity. The majority (56%) of IC neurons so identified received indirect central inputs (vagus alone: 25%; stellate ganglion alone: 27%; both: 48%). Fifty per cent transduced the cardiac milieu responding to multimodal stressors applied to the great vessels or heart. Fifty per cent of IC neurons exhibited cardiac cycle periodicity, with activity occurring primarily in late diastole into isovolumetric contraction. Cardiac-related activity in IC neurons was primarily related to direct cardiac mechano-sensory inputs and indirect autonomic efferent inputs. In response to mediastinal nerve stimulation, most IC neurons became excessively activated; such network behaviour preceded and persisted throughout AF. It was concluded that stochastic interactions occur among IC local circuit neuronal populations in the control of regional cardiac function. Modulation of IC local circuit neuronal recruitment may represent a novel approach for the treatment of cardiac disease, including atrial arrhythmias.
Figures







References
-
- Adams DJ, Cuevas J. Electrophysiological properties of intrinsic cardiac neurons. In: Armour JA, Ardell JL, editors. Basic and Clinical Neurocardiology. New York: Oxford University Press; 2004. pp. 1–60.
-
- Andresen MC, Kunze DL, Mendelowitz D. Central nervous system regulation of the heart. In: Armour JA, Ardell JL, editors. Basic and Clinical Neurocardiology. New York: Oxford University Press; 2004. pp. 187–219.
-
- Ardell JL. Intrathoracic neuronal regulation of cardiac function. In: Armour JA, Ardell JL, editors. Basic and Clinical Neurocardiology. New York: Oxford University Press; 2004. pp. 118–152.
-
- Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronic decentralized canine hearts. Am J Physiol Heart Circ Physiol. 1991;260:H713–H721. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

