Adhesins and host serum factors drive Yop translocation by yersinia into professional phagocytes during animal infection
- PMID: 23818844
- PMCID: PMC3688556
- DOI: 10.1371/journal.ppat.1003415
Adhesins and host serum factors drive Yop translocation by yersinia into professional phagocytes during animal infection
Abstract
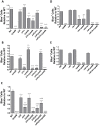
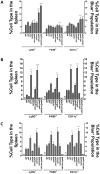
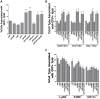
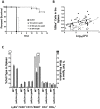
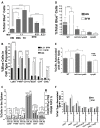
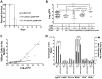
Yersinia delivers Yops into numerous types of cultured cells, but predominantly into professional phagocytes and B cells during animal infection. The basis for this cellular tropism during animal infection is not understood. This work demonstrates that efficient and specific Yop translocation into phagocytes by Yersinia pseudotuberculosis (Yptb) is a multi-factorial process requiring several adhesins and host complement. When WT Yptb or a multiple adhesin mutant strain, ΔailΔinvΔyadA, colonized tissues to comparable levels, ΔailΔinvΔyadA translocated Yops into significantly fewer cells, demonstrating that these adhesins are critical for translocation into high numbers of cells. However, phagocytes were still selectively targeted for translocation, indicating that other bacterial and/or host factors contribute to this function. Complement depletion showed that complement-restricted infection by ΔailΔinvΔyadA but not WT, indicating that adhesins disarm complement in mice either by prevention of opsonophagocytosis or by suppressing production of pro-inflammatory cytokines. Furthermore, in the absence of the three adhesins and complement, the spectrum of cells targeted for translocation was significantly altered, indicating that Yersinia adhesins and complement direct Yop translocation into neutrophils during animal infection. In summary, these findings demonstrate that in infected tissues, Yersinia uses adhesins both to disarm complement-dependent killing and to efficiently translocate Yops into phagocytes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
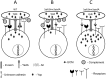
References
-
- Mota LJ, Cornelis GR (2005) The bacterial injection kit: type III secretion systems. Ann Med 37: 234–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5K12GM074869/GM/NIGMS NIH HHS/United States
- R25HL007785/HL/NHLBI NIH HHS/United States
- T32AI007422/AI/NIAID NIH HHS/United States
- UL1 TR000073/TR/NCATS NIH HHS/United States
- K12 GM074869/GM/NIGMS NIH HHS/United States
- R01 AI056068/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- AI056068/AI/NIAID NIH HHS/United States
- R56 AI073759/AI/NIAID NIH HHS/United States
- AI073759/AI/NIAID NIH HHS/United States
- R25 HL007785/HL/NHLBI NIH HHS/United States
- T32 AI007422/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

