Angiogenesis inhibitor bevacizumab increases the risk of ischemic heart disease associated with chemotherapy: a meta-analysis
- PMID: 23818962
- PMCID: PMC3688569
- DOI: 10.1371/journal.pone.0066721
Angiogenesis inhibitor bevacizumab increases the risk of ischemic heart disease associated with chemotherapy: a meta-analysis
Abstract
Concerns have arisen regarding the risk of ischemic heart disease with the novel antiangiogenic agent bevacizumab, a recombinant humanised monoclonal antibody to the vascular endothelial growth factor that is widely used in cancer treatment. Currently, the role of bevacizumab in ischemic heart disease is controversial. This meta-analysis was therefore performed to assess the overall risk of ischemic heart disease associated with the use of bevacizumab. The databases of PubMed, EMBASE and Web of Science were searched for English language studies of randomised controlled trials comparing bevacizumab with control therapy published through October 25, 2012. Summary incidence rates, relative risks (RRs) and 95% confidence intervals (CIs) were calculated using random-effects or fixed-effects models based on the heterogeneity of the included studies. A total of 4,617 patients from 7 randomised controlled trials were identified and included for analysis. Among those patients receiving bevacizumab, the summary incidence of ischemic heart disease was 1.0% (95% CI, 0.6%-1.4%). Patients treated with bevacizumab had a significantly increased risk of ischemic heart disease with an RR of 2.49 (95% CI, 1.37-4.52) compared with controls. In addition, both high doses and low doses of bevacizumab increased the risk of cardiac ischemia (low dose at 2.5 mg/kg per week: RR, 2.14 [95% CI, 1.09-4.19]; high dose at 5 mg/kg per week: RR, 4.81 [95% CI, 1.03-22.42]). Bevacizumab was also found to significantly increase the risk of cardiac ischemia in patients with colorectal cancer (RR, 2.13; 95% CI, 1.11-4.06) compared with controls. This meta-analysis shows the use of bevacizumab was associated with an increased risk of developing ischemic heart disease in colorectal cancer patients receiving this drug. Our conclusions are limited by the available data. Further evaluations of high-quality RCTs are needed.
Conflict of interest statement
Figures
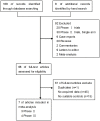


References
-
- Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1: 27–31. - PubMed
-
- Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23: 1011–1027. - PubMed
-
- Motzer RJ, Bukowski RM (2006) Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol 24: 5601–5608. - PubMed
-
- Jain RK, Duda DG, Clark JW, Loeffler JS (2006) Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol 3: 24–40. - PubMed
-
- Kerbel R, Folkman J (2002) Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2: 727–739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

