Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled Phase IIIb study
- PMID: 23819698
- PMCID: PMC3787813
- DOI: 10.3109/15412555.2013.814626
Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled Phase IIIb study
Abstract
Background: This randomized, double-blind, Phase IIIb study evaluated the 24-hour bronchodilatory efficacy of aclidinium bromide versus placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD).
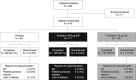
Methods: Patients received aclidinium 400 μg twice daily (morning and evening), tiotropium 18 μg once daily (morning), or placebo for 6 weeks. The primary endpoint was change from baseline in forced expiratory volume in 1 second area under the curve for the 24-hour period post-morning dose (FEV1 AUC0-24) at week 6. Secondary and additional endpoints included FEV1 AUC12-24, COPD symptoms (EXAcerbations of chronic pulmonary disease Tool-Respiratory Symptoms [E-RS] total score and additional symptoms questionnaire), and safety.
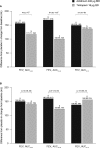
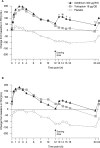
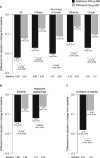
Results: Overall, 414 patients were randomized and treated (FEV1 1.63 L [55.8% predicted]). Compared with placebo, FEV1 AUC0-24 and FEV1 AUC12-24 were significantly increased from baseline with aclidinium (∆ = 150 mL and 160 mL, respectively; p < 0.0001) and tiotropium (∆ = 140 mL and 123 mL, respectively; p < 0.0001) at week 6. Significant improvements in E-RS total scores over 6 weeks were numerically greater with aclidinium (p < 0.0001) than tiotropium (p < 0.05) versus placebo. Only aclidinium significantly reduced the severity of early-morning cough, wheeze, shortness of breath, and phlegm, and of nighttime symptoms versus placebo (p < 0.05). Adverse-event (AE) incidence (28%) was similar between treatments. Few anticholinergic AEs (<1.5%) or serious AEs (<3%) occurred in any group.
Conclusions: Aclidinium provided significant 24-hour bronchodilation versus placebo from day 1 with comparable efficacy to tiotropium after 6 weeks. Improvements in COPD symptoms were consistently numerically greater with aclidinium versus tiotropium. Aclidinium was generally well tolerated.
Figures

References
-
- Postma DS, KoÎter GH, vd Mark TW, Reig RP, Sluiter HJ. The effects of oral slow-release terbutaline on the circadian variation in spirometry and arterial blood gas levels in patients with chronic airflow obstruction. Chest. 1985;87:653–657. - PubMed
-
- van Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, Cornelissen PJ. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006;129:509–517. - PubMed
-
- Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D, Ostinelli J. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37:264–272. - PubMed
-
- Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin. 2009;25:2043–2048. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
