The first engagement of partners in the Euprymna scolopes-Vibrio fischeri symbiosis is a two-step process initiated by a few environmental symbiont cells
- PMID: 23819708
- PMCID: PMC3937295
- DOI: 10.1111/1462-2920.12179
The first engagement of partners in the Euprymna scolopes-Vibrio fischeri symbiosis is a two-step process initiated by a few environmental symbiont cells
Abstract
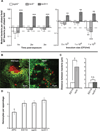
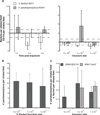
We studied the Euprymna scolopes-Vibrio fischeri symbiosis to characterize, in vivo and in real time, the transition between the bacterial partner's free-living and symbiotic life styles. Previous studies using high inocula demonstrated that environmental V. fischeri cells aggregate during a 3 h period in host-shed mucus along the light organ's superficial ciliated epithelia. Under lower inoculum conditions, similar to the levels of symbiont cells in the environment, this interaction induces haemocyte trafficking into these tissues. Here, in experiments simulating natural conditions, microscopy revealed that at 3 h following first exposure, only ∼ 5 V. fischeri cells aggregated on the organ surface. These cells associated with host cilia and induced haemocyte trafficking. Symbiont viability was essential and mutants defective in symbiosis initiation and/or production of certain surface features, including the Mam7 protein, which is implicated in host cell attachment of V. cholerae, associated normally with host cilia. Studies with exopolysaccharide mutants, which are defective in aggregation, suggest a two-step process of V. fischeri cell engagement: association with host cilia followed by aggregation, i.e. host cell-symbiont interaction with subsequent symbiont-symbiont cell interaction. Taken together, these data provide a new model of early partner engagement, a complex model of host-symbiont interaction with exquisite sensitivity.
© 2013 Society for Applied Microbiology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Adam EC, Mitchell BS, Schumacher DU, Grant G, Schumacher U. Pseudomonas aeruginosa II lectin stops human ciliary beating: therapeutic implications of fucose. Am J Respir Crit Care Med. 1997;155:2102–2104. - PubMed
-
- Anderton TL, Maskell DJ, Preston A. Ciliostasis is a key early event during colonization of canine tracheal tissue by Bordetella bronchiseptica. Microbiology. 2004;150:2843–2855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

