Uncoupling protein 2 impacts endothelial phenotype via p53-mediated control of mitochondrial dynamics
- PMID: 23819990
- PMCID: PMC3869454
- DOI: 10.1161/CIRCRESAHA.113.301319
Uncoupling protein 2 impacts endothelial phenotype via p53-mediated control of mitochondrial dynamics
Abstract
Rationale: Mitochondria, although required for cellular ATP production, are also known to have other important functions that may include modulating cellular responses to environmental stimuli. However, the mechanisms whereby mitochondria impact cellular phenotype are not yet clear.
Objective: To determine how mitochondria impact endothelial cell function.
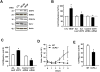
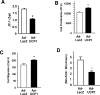
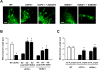
Methods and results: We report here that stimuli for endothelial cell proliferation evoke strong upregulation of mitochondrial uncoupling protein 2 (UCP2). Analysis in silico indicated increased UCP2 expression is common in highly proliferative cell types, including cancer cells. Upregulation of UCP2 was critical for controlling mitochondrial membrane potential (Δψ) and superoxide production. In the absence of UCP2, endothelial growth stimulation provoked mitochondrial network fragmentation and premature senescence via a mechanism involving superoxide-mediated p53 activation. Mitochondrial network fragmentation was both necessary and sufficient for the impact of UCP2 on endothelial cell phenotype.
Conclusions: These data identify a novel mechanism whereby mitochondria preserve normal network integrity and impact cell phenotype via dynamic regulation of UCP2.
Keywords: angiogenesis; endothelial function; endothelium; ischemia; mitochondria; mitochondrial uncoupling proteins; superoxides.
Conflict of interest statement
Figures









Comment in
-
Do not overcharge the system or it will explode!: how mitochondrial uncoupling protein 2 maintains endothelial function.Circ Res. 2013 Sep 13;113(7):846-8. doi: 10.1161/CIRCRESAHA.113.302127. Circ Res. 2013. PMID: 24030018 No abstract available.
Similar articles
-
Increased activity of mitochondrial uncoupling protein 2 improves stress resistance in cultured endothelial cells exposed in vitro to high glucose levels.Am J Physiol Heart Circ Physiol. 2015 Jul 1;309(1):H147-56. doi: 10.1152/ajpheart.00759.2014. Epub 2015 Apr 24. Am J Physiol Heart Circ Physiol. 2015. PMID: 25910810
-
Respiratory uncoupling by UCP1 and UCP2 and superoxide generation in endothelial cell mitochondria.Am J Physiol Endocrinol Metab. 2005 Jan;288(1):E71-9. doi: 10.1152/ajpendo.00332.2004. Epub 2004 Aug 31. Am J Physiol Endocrinol Metab. 2005. PMID: 15339748
-
Do not overcharge the system or it will explode!: how mitochondrial uncoupling protein 2 maintains endothelial function.Circ Res. 2013 Sep 13;113(7):846-8. doi: 10.1161/CIRCRESAHA.113.302127. Circ Res. 2013. PMID: 24030018 No abstract available.
-
New aspects of mitochondrial Uncoupling Proteins (UCPs) and their roles in tumorigenesis.Int J Mol Sci. 2011;12(8):5285-93. doi: 10.3390/ijms12085285. Epub 2011 Aug 17. Int J Mol Sci. 2011. PMID: 21954358 Free PMC article. Review.
-
Uncoupling protein UCP2: when mitochondrial activity meets immunity.FEBS Lett. 2010 Apr 16;584(8):1437-42. doi: 10.1016/j.febslet.2010.03.014. Epub 2010 Mar 15. FEBS Lett. 2010. PMID: 20227410 Review.
Cited by
-
PGC-1α dictates endothelial function through regulation of eNOS expression.Sci Rep. 2016 Dec 2;6:38210. doi: 10.1038/srep38210. Sci Rep. 2016. PMID: 27910955 Free PMC article.
-
Exercise-mediated wall shear stress increases mitochondrial biogenesis in vascular endothelium.PLoS One. 2014 Nov 6;9(11):e111409. doi: 10.1371/journal.pone.0111409. eCollection 2014. PLoS One. 2014. PMID: 25375175 Free PMC article.
-
Mitochondria in endothelial cells angiogenesis and function: current understanding and future perspectives.J Transl Med. 2023 Jul 5;21(1):441. doi: 10.1186/s12967-023-04286-1. J Transl Med. 2023. PMID: 37407961 Free PMC article. Review.
-
Endothelial Cells Mediated by UCP2 Control the Neurogenic-to-Astrogenic Neural Stem Cells Fate Switch During Brain Development.Adv Sci (Weinh). 2022 Jun;9(18):e2105208. doi: 10.1002/advs.202105208. Epub 2022 Apr 30. Adv Sci (Weinh). 2022. PMID: 35488517 Free PMC article.
-
Mitochondria, endothelial cell function, and vascular diseases.Front Physiol. 2014 May 6;5:175. doi: 10.3389/fphys.2014.00175. eCollection 2014. Front Physiol. 2014. PMID: 24834056 Free PMC article. Review.
References
-
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. - PubMed
-
- Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. - PubMed
-
- Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763:723–736. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

