Estimates of the timing of reductions in genital warts and high grade cervical intraepithelial neoplasia after onset of human papillomavirus (HPV) vaccination in the United States
- PMID: 23820080
- PMCID: PMC6727206
- DOI: 10.1016/j.vaccine.2013.06.050
Estimates of the timing of reductions in genital warts and high grade cervical intraepithelial neoplasia after onset of human papillomavirus (HPV) vaccination in the United States
Abstract
Background: The objective of this study was to estimate the number of years after onset of a quadrivalent HPV vaccination program before notable reductions in genital warts and cervical intraepithelial neoplasia (CIN) will occur in teenagers and young adults in the United States.
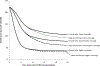
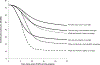
Methods: We applied a previously published model of HPV vaccination in the United States and focused on the timing of reductions in genital warts among both sexes and reductions in CIN 2/3 among females. Using different coverage scenarios, the lowest being consistent with current 3-dose coverage in the United States, we estimated the number of years before reductions of 10%, 25%, and 50% would be observed after onset of an HPV vaccination program for ages 12-26 years.
Results: The model suggested female-only HPV vaccination in the intermediate coverage scenario will result in a 10% reduction in genital warts within 2-4 years for females aged 15-19 years and a 10% reduction in CIN 2/3 among females aged 20-29 years within 7-11 years. Coverage had a major impact on when reductions would be observed. For example, in the higher coverage scenario a 25% reduction in CIN2/3 would be observed with 8 years compared with 15 years in the lower coverage scenario.
Conclusions: Our model provides estimates of the potential timing and magnitude of the impact of HPV vaccination on genital warts and CIN 2/3 at the population level in the United States. Notable, population-level impacts of HPV vaccination on genital warts and CIN 2/3 can occur within a few years after onset of vaccination, particularly among younger age groups. Our results are generally consistent with early reports of declines in genital warts among youth.
Keywords: Cervical neoplasms; Genital warts; Models; Papillomavirus; Vaccination.
Published by Elsevier Ltd.
Conflict of interest statement
Figures

Similar articles
-
Impact of HPV vaccination on the hospitalizations for anogenital warts and high-grade cervical intraepithelial neoplasia in Brazil: A national analysis.Hum Vaccin Immunother. 2025 Dec;21(1):2514949. doi: 10.1080/21645515.2025.2514949. Epub 2025 Jun 15. Hum Vaccin Immunother. 2025. PMID: 40518563 Free PMC article.
-
The impact of Germany's human papillomavirus immunization program on HPV-related anogenital diseases: a retrospective analysis of claims data from statutory health insurances.Arch Gynecol Obstet. 2024 Nov;310(5):2639-2646. doi: 10.1007/s00404-024-07692-y. Epub 2024 Sep 4. Arch Gynecol Obstet. 2024. PMID: 39230793 Free PMC article.
-
Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis.Lancet. 2019 Aug 10;394(10197):497-509. doi: 10.1016/S0140-6736(19)30298-3. Epub 2019 Jun 26. Lancet. 2019. PMID: 31255301 Free PMC article.
-
High prevalence of genital HPV infection among long-term monogamous partners of women with cervical dysplasia or genital warts-Another reason for HPV vaccination of boys.Dermatol Ther. 2017 Jan;30(1). doi: 10.1111/dth.12435. Epub 2016 Oct 9. Dermatol Ther. 2017. PMID: 27723194
-
Population impact of HPV vaccines: summary of early evidence.J Adolesc Health. 2013 Dec;53(6):679-82. doi: 10.1016/j.jadohealth.2013.09.018. J Adolesc Health. 2013. PMID: 24263069 Free PMC article. Review.
Cited by
-
Impact and Cost-effectiveness of 3 Doses of 9-Valent Human Papillomavirus (HPV) Vaccine Among US Females Previously Vaccinated With 4-Valent HPV Vaccine.J Infect Dis. 2016 Jun 1;213(11):1694-700. doi: 10.1093/infdis/jiw046. Epub 2016 Feb 9. J Infect Dis. 2016. PMID: 26908738 Free PMC article.
-
Regional variations in HPV vaccination among 9-17 year old adolescent females from the BRFSS, 2008-2010.Hum Vaccin Immunother. 2014;10(12):3475-83. doi: 10.4161/21645515.2014.980202. Hum Vaccin Immunother. 2014. PMID: 25668660 Free PMC article.
-
Cost-effectiveness of nonavalent HPV vaccination among males aged 22 through 26 years in the United States.Vaccine. 2018 Jul 5;36(29):4362-4368. doi: 10.1016/j.vaccine.2018.04.071. Vaccine. 2018. PMID: 29887325 Free PMC article.
-
The cost-effectiveness of human papillomavirus vaccine catch-up programs for women.J Infect Dis. 2015 Jan 15;211(2):172-4. doi: 10.1093/infdis/jiu414. Epub 2014 Jul 23. J Infect Dis. 2015. PMID: 25057043 Free PMC article. No abstract available.
-
Human papillomavirus genotype-specific prevalence across the continuum of cervical neoplasia and cancer.Cancer Epidemiol Biomarkers Prev. 2015 Jan;24(1):230-40. doi: 10.1158/1055-9965.EPI-14-0775. Epub 2014 Nov 2. Cancer Epidemiol Biomarkers Prev. 2015. PMID: 25363635 Free PMC article.
References
-
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(RR-2):1–24. - PubMed
-
- Garnett GP, Kim JJ, French K, Goldie SJ. Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006;24(Suppl. 3):S178–86. - PubMed
-
- Brisson M, Van d V, De WP, Boily MC. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine 2007;25(July (20)):5399–408. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

