Envelope protein dynamics in paramyxovirus entry
- PMID: 23820396
- PMCID: PMC3705453
- DOI: 10.1128/mBio.00413-13
Envelope protein dynamics in paramyxovirus entry
Abstract
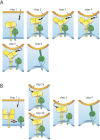
Paramyxoviruses include major pathogens with significant global health and economic impact. This large family of enveloped RNA viruses infects cells by employing two surface glycoproteins that tightly cooperate to fuse their lipid envelopes with the target cell plasma membrane, an attachment and a fusion (F) protein. Membrane fusion is believed to depend on receptor-induced conformational changes within the attachment protein that lead to the activation and subsequent refolding of F. While structural and mechanistic studies have considerably advanced our insight into paramyxovirus cell adhesion and the structural basis of F refolding, how precisely the attachment protein links receptor engagement to F triggering remained poorly understood. Recent reports based on work with several paramyxovirus family members have transformed our understanding of the triggering mechanism of the membrane fusion machinery. Here, we review these recent findings, which (i) offer a broader mechanistic understanding of the paramyxovirus cell entry system, (ii) illuminate key similarities and differences between entry strategies of different paramyxovirus family members, and (iii) suggest new strategies for the development of novel therapeutics.
Figures

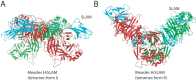
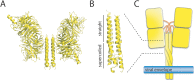
References
-
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, Ksiazek TG, Rollin PE, Zaki SR, Shieh W, Goldsmith CS, Gubler DJ, Roehrig JT, Eaton B, Gould AR, Olson J, Field H, Daniels P, Ling AE, Peters CJ, Anderson LJ, Mahy BW. 2000. Nipah virus: a recently emergent deadly paramyxovirus. Science 288:1432–1435 - PubMed
-
- Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L, Westbury H, Hiley L, Selvey L, Rodwell B, Ketterer P. 1995. A morbillivirus that caused fatal disease in horses and humans. Science 268:94–97 - PubMed
-
- Lamb RA, Parks GD. 2007. Paramyxoviridae: the viruses and their replication, p 1449–1496 In Knipe DM, Howley PM, Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
