The GlycoFilter: a simple and comprehensive sample preparation platform for proteomics, N-glycomics and glycosylation site assignment
- PMID: 23820512
- PMCID: PMC3790305
- DOI: 10.1074/mcp.M113.027953
The GlycoFilter: a simple and comprehensive sample preparation platform for proteomics, N-glycomics and glycosylation site assignment
Abstract
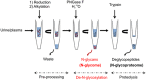
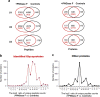
Current strategies to study N-glycoproteins in complex samples are often discrete, focusing on either N-glycans or N-glycosites enriched by sugar-based techniques. In this study we report a simple and rapid sample preparation platform, the GlycoFilter, which allows a comprehensive characterization of N-glycans, N-glycosites, and proteins in a single workflow. Both PNGase F catalyzed de-N-glycosylation and trypsin digestions are accelerated by microwave irradiation and performed sequentially in a single spin filter. Both N-glycans and peptides (including de-N-glycosylated peptides) are separately collected by filtration. The condition to effectively collect complex and heterogeneous N-glycans was established on model glycoproteins, bovine ribonuclease B, bovine fetuin, and human serum IgG. With this platform, the N-glycome, N-glycoproteome and proteome of human urine and plasma were characterized. Overall, a total of 865 and 295 N-glycosites were identified from three pairs of urine and plasma samples, respectively. Many sites were defined unambiguously as partially occupied by the detection of their nonsugar-modified peptides (128 from urine and 61 from plasma), demonstrating that partial occupancy of N-glycosylation occurs frequently. Given the likely high prevalence and variability of partial occupancy, glycoprotein quantification based exclusively on deglycosylated peptides may lead to inaccurate quantification.
Figures


References
-
- Apweiler R., Hermjakob H., Sharon N. (1999) On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys.. Acta 1473, 4–8 - PubMed
-
- Stanley P., Schachter H., Taniguchi N. (2009) N-Glycans. In: Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E., eds. Essentials of Glycobiology, 2nd Ed., Cold Spring Harbor; (NY) - PubMed
-
- Mariño K., Bones J., Kattla J. J., Rudd P. M. (2010) A systematic approach to protein glycosylation analysis: a path through the maze. Nat. Chem. Biol. 6, 713–723 - PubMed
-
- Bosques C. J., Collins B. E., Meador J. W., 3rd, Sarvaiya H., Murphy J. L., Dellorusso G., Bulik D. A., Hsu I. H., Washburn N., Sipsey S. F., Myette J. R., Raman R., Shriver Z., Sasisekharan R., Venkataraman G. (2010) Chinese hamster ovary cells can produce galactose-alpha-1,3-galactose antigens on proteins. Nat. Biotechnol. 28, 1153–1156 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

