Striatal shape in Parkinson's disease
- PMID: 23820588
- PMCID: PMC3742686
- DOI: 10.1016/j.neurobiolaging.2013.05.017
Striatal shape in Parkinson's disease
Abstract
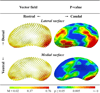
Parkinson's disease (PD) is marked pathologically by nigrostriatal dopaminergic terminal loss. Histopathological and in vivo labeling studies demonstrate that this loss occurs most extensively in the caudal putamen and caudate head. Previous structural studies have suggested reduced striatal volume and atrophy of the caudate head in PD subjects. The spatial distribution of atrophy in the putamen, however, has not been characterized. We aimed to delineate the specific locations of atrophy in both of these striatal structures. T1- and T2-weighted brain MR (3T) images were obtained from 40 PD and 40 control subjects having no dementia and similar age and gender distributions. Shape analysis was performed using doubly segmented regions of interest. Compared to controls, PD subjects had lower putamen (p = 0.0003) and caudate (p = 0.0003) volumes. Surface contraction magnitudes were greatest on the caudal putamen (p ≤ 0.005) and head and dorsal body of the caudate (p ≤ 0.005). This spatial distribution of striatal atrophy is consistent with the known pattern of dopamine depletion in PD and may reflect global consequences of known cellular remodeling phenomena.
Keywords: Caudate; Magnetic resonance imaging (MRI); Morphology; Parkinson's disease; Putamen; Shape; Striatum; Structure; Volume.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures



References
-
- UNC NeuroImage Analysis Lab Manual. [Accessed 5-15-2012];IBIS Network. 2007 Online: http://ibis-network.org/unc/mri/roiprotocols.htm.
-
- Bezard E, et al. Treating Parkinson's disease: preserve the spines!(Commentary on Soderstrom) Eur. J. Neurosci. 2010;31(3):477. - PubMed
-
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease Neurobiol. Aging. 2003;24(2):197–211. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

