The Alzheimer's β-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques
- PMID: 23820808
- PMCID: PMC3753469
- DOI: 10.1007/s00401-013-1152-3
The Alzheimer's β-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques
Erratum in
- Acta Neuropathol. 2013 Oct;126(4):623
Abstract
β-Site amyloid precursor protein (APP) cleaving enzyme-1 (BACE1) is the β-secretase that initiates Aβ production in Alzheimer's disease (AD). BACE1 levels are increased in AD, which could contribute to pathogenesis, yet the mechanism of BACE1 elevation is unclear. Furthermore, the normal function of BACE1 is poorly understood. We localized BACE1 in the brain at both the light and electron microscopic levels to gain insight into normal and pathophysiologic roles of BACE1 in health and AD, respectively. Our findings provide the first ultrastructural evidence that BACE1 localizes to vesicles (likely endosomes) in normal hippocampal mossy fiber terminals of both non-transgenic and APP transgenic (5XFAD) mouse brains. In some instances, BACE1-positive vesicles were located near active zones, implying a function for BACE1 at the synapse. In addition, BACE1 accumulated in swollen dystrophic autophagosome-poor presynaptic terminals surrounding amyloid plaques in 5XFAD cortex and hippocampus. Importantly, accumulations of BACE1 and APP co-localized in presynaptic dystrophies, implying increased BACE1 processing of APP in peri-plaque regions. In primary cortical neuron cultures, treatment with the lysosomal protease inhibitor leupeptin caused BACE1 levels to increase; however, exposure of neurons to the autophagy inducer trehalose did not reduce BACE1 levels. This suggests that BACE1 is degraded by lysosomes but not by autophagy. Our results imply that BACE1 elevation in AD could be linked to decreased lysosomal degradation of BACE1 within dystrophic presynaptic terminals. Elevated BACE1 and APP levels in plaque-associated presynaptic dystrophies could increase local peri-plaque Aβ generation and accelerate amyloid plaque growth in AD.
Figures
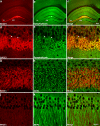


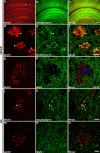




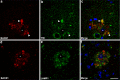

References
-
- Adalbert R, Nogradi A, Babetto E, Janeckova L, Walker SA, Kerschensteiner M, Misgeld T, Coleman MP. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain J Neurol. 2009;132(Pt 2):402–416. - PubMed
-
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195(1):51–86. - PubMed
-
- Bi X, Zhou J, Lynch G. Lysosomal protease inhibitors induce mega neurites and tangle-like structures in entorhinohippocampal regions vulnerable to Alzheimer’s disease. Exp Neurol. 1999;158(2):312–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

